Figure 1.
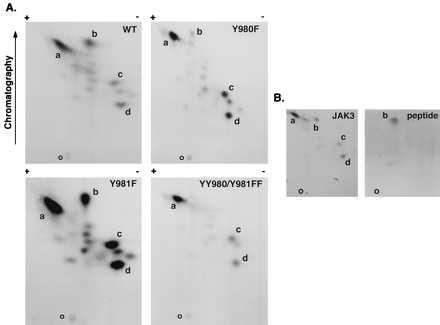
Tryptic phosphopeptide mapping of autophosphorylated JAK3. WT JAK3, mutant Y980F, mutant Y981F, and mutant YY980/981FF were expressed in COS-7 cells and immunoprecipitated using anti-JAK3 C-terminal antiserum, and in vitro kinase assays were performed for 10 min at room temperature. The phosphoproteins were subjected to SDS/PAGE, transferred to nitrocellulose, and exposed to x-ray film. The portions of the membrane containing WT and mutant JAK3 proteins were excised and digested in situ with trypsin. Eluted tryptic phosphopeptides from WT and indicated mutants (A) or eluted tryptic peptides from WT JAK3 mixed with a synthetic phosphopeptide DYpYpVVR (B) were analyzed by two-dimensional peptide mapping. The 32P-labeled phosphopeptides were visualized by autoradiography (B Left), and the position of synthetic phosphopeptide was determined by ninhydrin staining as indicated (B Right). The orientation of the positive and negative electrodes during electrophoresis is indicated along with the direction of chromatography; the origin is indicated by the letter O. The most prominent spots were designated a–d. Because of the effect of the mutations on kinase activity, the exposure time was varied to achieve similar intensities for the map of each mutant (exposure time: Y980F, 96 hr; YY980/981FF, 48 hr; WT JAK3, 16 hr; and Y981F, 6 hr).
