FIGURE 2.
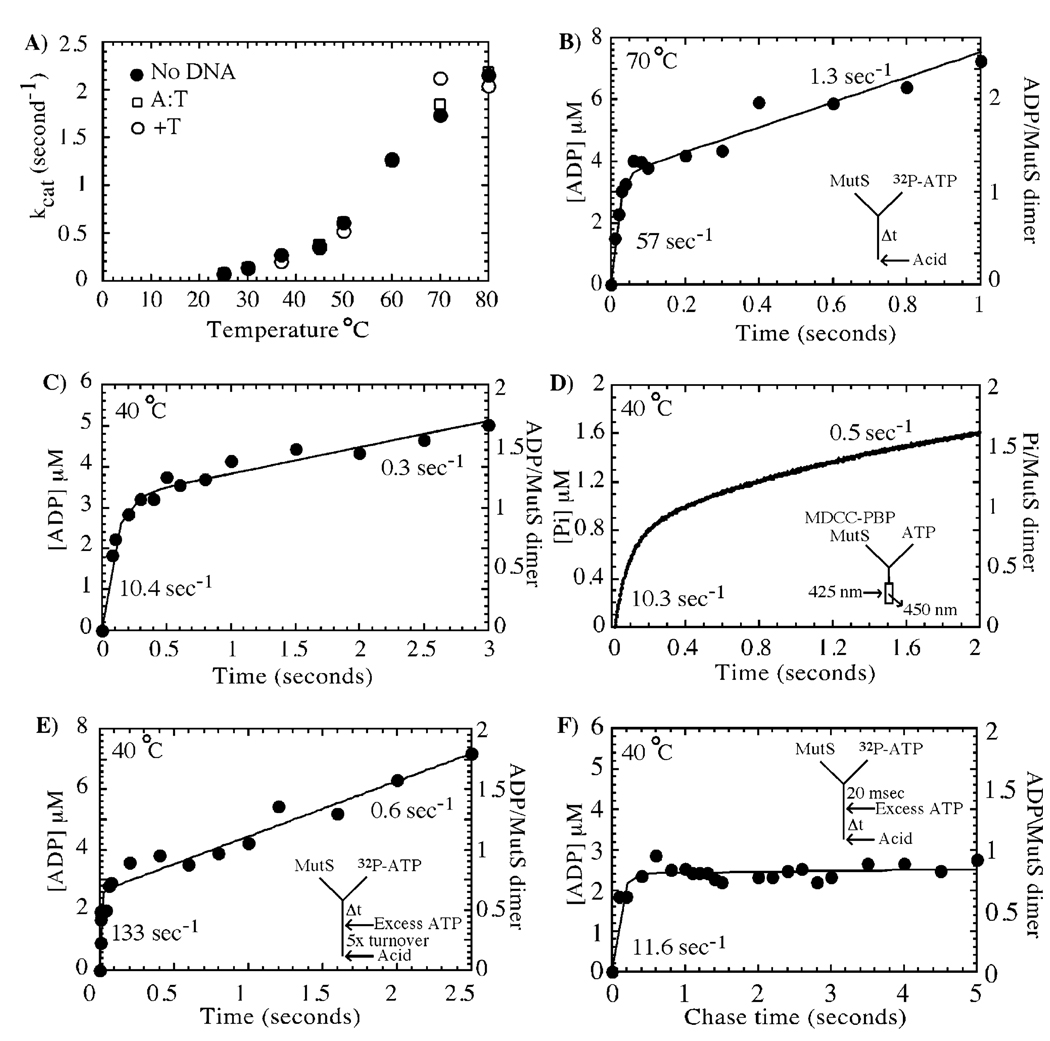
Asymmetry in ATP binding is recapitulated during ATP hydrolysis. In panel A, steady-state ATPase activity of T. aquaticus MutS is temperature-dependent with kcat approaching a maximum of 2 s−1 at 80 °C. In panel B, pre-steady-state rapid-quench analysis of MutS activity at 70 °C reveals a burst of ATP hydrolysis at a rate of 57 ± 11 s−1 and amplitude of 3.5 ±0.15 µM, followed by a slow steady-state phase at 1.3 ± 0.12 s−1 (3 µM MutS dimer and 500 µM ATP in the reaction). The burst phase is equivalent to rapid hydrolysis of one ATP molecule per MutS dimer. In panel C, the burst rate and kcat vary with temperature (e.g., at 40 °C the burst rate constant is 10.4 s−1 and the linear rate constant is 0.3 s−1, although the amplitude is still 3.2 µM, see Table 1). In panel D, a fluorescent reporter assay measures a burst of phosphate release from 1 µM MutS at 10.3 ± 0.3 s−1 at 40 °C and 1.1 ± 0.02 µM amplitude (one ADP/Pi per MutS dimer), suggesting that the phosphate is released rapidly following ATP hydrolysis. In panel E, pulse–chase experiments at 40 °C with 3 µM MutS and 500 µM ATP also show a burst amplitude of one ADP per dimer and yield an ATP binding rate constant of 0.25 ± 0.1 µM−1 s−1. In panel F, a pulse–chase time experiment, in which hydrolysis of 32P-ATP (500 µM) bound to MutS (3 µM dimer) prior to addition of unlabeled ATP chase (10 mM) is observed over time, detects only one ADP formed per MutS dimer in the first turnover (burst rate = 11.6 ± 2.3 s−1).