FIGURE 3.
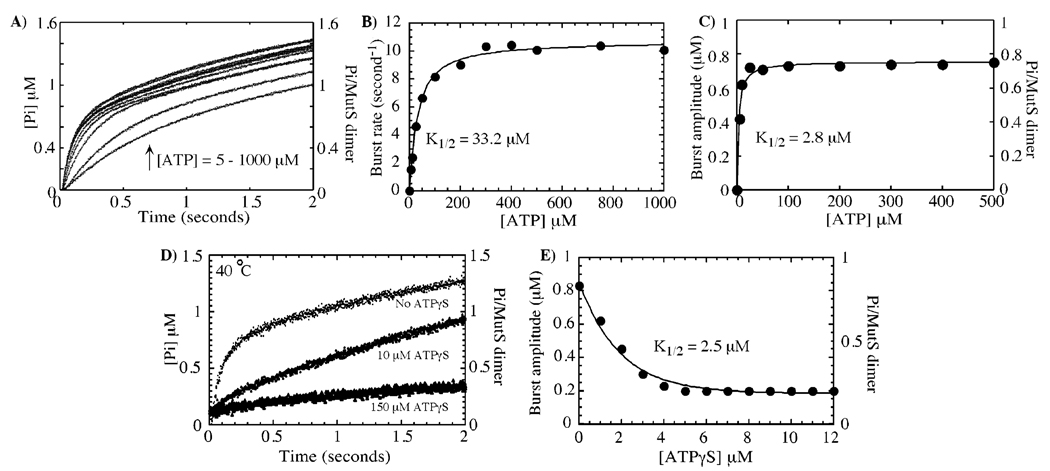
The high-affinity ATP-binding site on MutS catalyzes rapid ATP hydrolysis. In panel A, phosphate-release kinetics were measured at increasing ATP concentrations and, in panel B, yielded a maximum burst rate constant of 10.7 ± 0.2 s−1 and K1/2 = 33.2 ± 2.5 µM, as well as, in panel C, a maximum amplitude of 0.8 ± 0.01 µM and K1/2 = 2.8 ± 0.4 µM for 1 µM MutS dimer at 40 °C. In panel D, preincubation of MutS (1 µM dimer) with increasing concentrations of ATPγS (0–200 µM) suppresses the rapid burst of hydrolysis (500 µM ATP in the reaction). In panel E, no burst phase can be detected at ATPγS concentrations higher than 5 µM; that is, following ATPγS binding to the high-affinity site (see Figure 1A), and a plot of decreasing burst amplitudes versus ATPγS concentration fit to a hyperbola yields K1/2 = 2.5 ± 0.7 µM (similar to the Kd for ATPγS binding to the high-affinity site on MutS, see Figure 1A).