Figure 8.
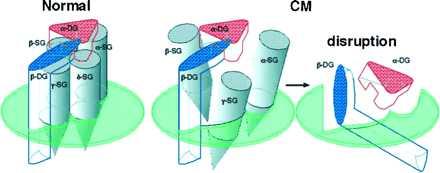
Hypothetical model of the DAGC architecture in normal cardiac sarcolemma and its disruption caused by deficiency of δ-SG in CM hamsters. The horizontal planes indicate sarcolemma, above which is the extracellular space. α-DG directly binds extracellular matrix laminin (14) and β-DG directly binds dystrophin with the C-terminal intracellular domain (25). (Left) In normal hamsters, the four SGs bind one another at the extracellular domains and constitute the SG subcomplex, which serves as a molecular stabilizer for the DG subcomplex. (Center and Right) Deficiency of δ-SG in the CM hamsters could disrupt the SG subcomplex (Center) because, for example, α-SG does not bind to γ-SG, and eventually the whole DAGC (Right), rendering cardiomyocytes more susceptible to mechanical stress generated by contraction of cardiac muscle. Molecular weights of α-, β-, γ-, and δ-SGs and α- and β-DGs are 50, 43, 35, 35, 156, and 43 kDa, respectively (7–10, 12, 14).
