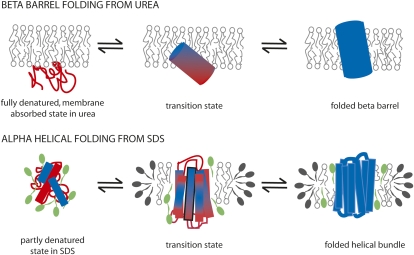
Fig. 1.
Schematic diagrams of proposed folding models for β-barrel and α-helical membrane proteins, highlighting potential transition state structures from φ-value studies. Folding of a β-barrel protein occurs from a fully denatured, membrane-absorbed state in urea with a tilted, partly inserted transition state as proposed by Huysmans et al. (1). In contrast, folding of an α-helical protein such as bacteriorhodopsin occurs from a partly denatured state in SDS, which contains some helical content. The transition state is proposed to have significant native helix content. Only one transmembrane helix has been analyzed by φ-values (3), and this suggests a largely formed helix with partial helix formation at the cytoplasmic side (shown in the bottom of this diagram, with this helix outlined in black). The degree of structure in other helices is estimated from previous studies (e.g., ref 15). Unfolded structure is shown in red and folded structure in blue. SDS is shown in green. PagP was folded into lipid bilayers vesicles, whereas bacteriorhodopsin was folded into mixed DMPC/CHAPS micelles (the detergent CHAPS is shown here capping the edges of a DMPC disc).