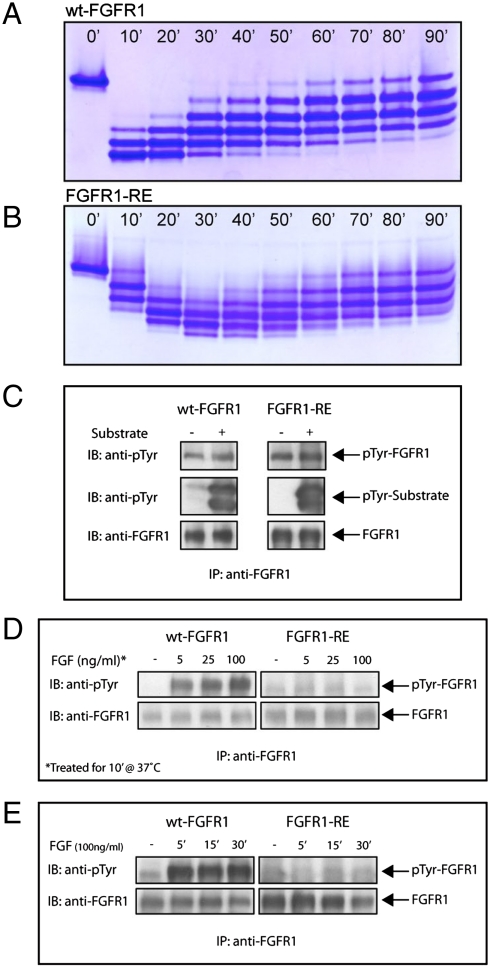
Fig. 3.
Autophosphorylation of FGFR1 in vitro and in vivo. Profiles of in vitro phosphorylation reactions of isolated kinase domains of (A) wt-FGFR1 and (B) FGFR1-RE at room temperature as a function of time. (C) Kinase activity of FGFR1-RE in vitro is maintained. Lysates of L6 cells expressing wt-FGFR1 or the FGFR1-RE mutant were subjected to immunoprecipitation with anti-FGFR1 antibodies. The immunoprecipitates were then incubated in the presence or absence of an FGFR1 substrate (PLCγ fragment, described in the results) for 30 min at room temperature followed by SDS-PAGE and immunoblotting with anti-pTyr or anti-FGFR1 antibodies. (D) Autophosphorylation of FGFR1-RE in vivo, is strongly compromised. L6 cells expressing either wt-FGFR1 or its RE mutant were stimulated with increasing concentrations of FGF (as indicated) for 10 min at 37 °C. Lysates of unstimulated or FGF-stimulated cells were subjected to immunoprecipitation using anti-FGFR1 antibodies followed by SDS-PAGE and immunoblotting with antipTyr or anti FGFR1 antibodies. (E) L6 cells expressing wt-FGFR1 or FGFR1-RE were stimulated with 100 ng/ml FGF for different times (as indicated). Lysates of unstimulated or FGF stimulated cells were subjected to SDS-PAGE followed by immunoblotting with anti-pTyr or anti-FGFR1 antibodies.