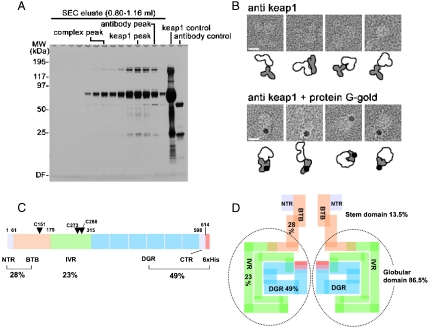
Fig. 6.
BTB, IVR, and DC domains of Keap1.(A) Purification of the anti-DC antibody-bound Keap1 complex. SDS-PAGE analysis shows isolation of the antibody/Keap1 complex by SEC. The purified complex was eluted in early fractions at approximately 290-kDa region. (B) The large cherries of the molecule contain the DC domains. Gallery of negatively stained anti-DC/Keap1 complexes (Upper) and protein-G-gold/anti-DC antibody/Keap1 complexes (Bottom). Schematic diagrams of Keap1 (White), antibodies (Gray), and gold particles (Black) are illustrated below each panel. Scale bars,100 Å. (C) Protein architecture of Keap1 showing BTB, IVR, DGR, and CTR domains with corresponding primary sequence boundaries. Reactive cysteines (Cys151, Cys273, and Cys288) important for stress sensing are indicated with single letter codes and residue numbers. Percentages (%) of each functional domain are also shown. (D) Schematic distribution of the four functional domains in the Keap1 homodimer, as predicted by volume ratio. The stem structure occupies only 13.5% of the whole volume, while the globular regions comprise about 86.5%, suggesting that the entire IVR and part of the BTB domain are integrated into the globular cherries.