Fig. 1.
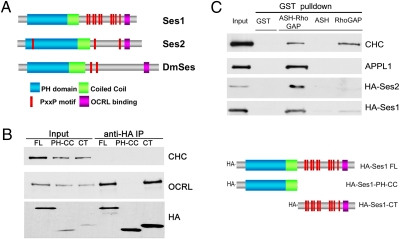
(A) Domain cartoon of Ses proteins. Vertebrates have two Ses genes, Ses1/FAM109a and Ses2/Fam109b, whereas Drosophila (DmSes, CG12393) and all invertebrates examined to date have a single Ses gene. Ses proteins harbor a PH domain immediately followed by a putative coiled-coil region and then by a predicted unfolded C-terminal portion containing several PxxP motifs and a short highly conserved sequence (OCRL-binding site) close to the C terminus. (B ) The C-terminal portion of Ses1 is necessary and sufficient for OCRL binding. HA-tagged fragments of Ses1 (Right) were overexpressed in Cos7 cells and immunoprecipitated (IP) from cell extracts (Input) with antibodies directed against the HA tag. The Western blots shown (Left) demonstrate that endogenous OCRL coprecipitated with both full-length (FL) Ses1 and the C-terminal (CT) region of Ses1. Clathrin heavy chain (CHC) is used as a negative control. (C) Western blots of GST pulldowns from Cos7 cells expressing either HA-tagged human Ses1 or HA-tagged Ses2. Both HA-Ses1 and HA-Ses2, like endogenous APPL1 (20), strongly interact with a GST fusion of the ASH-RhoGAP–like domain of OCRL but not with the GST-ASH domain alone, the GST-RhoGAP–like domain alone, or GST. Both the ASH-RhoGAP–like domain and the RhoGAP–like domain alone bind clathrin, as expected (20).