Fig. 4.
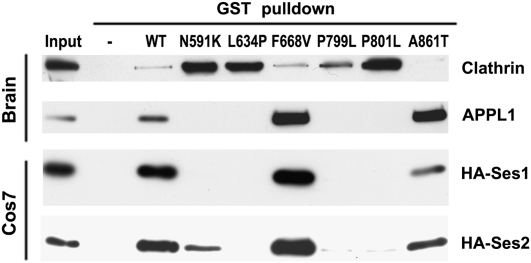
APPL1 and Ses1 binding to the ASH-RhoGAP–like domain is similarly abolished in a subset of mutations that cause Lowe syndrome. Western blots of GST pulldowns from mouse brain extracts (APPL1 and clathrin; Upper Panels) and transfected Cos7 cells (HA-Ses1 and HA-Ses2; Lower Panels) on GST fusions of WT and mutant human ASH-RhoGAP–like domain. In common with all other Lowe syndrome mutations of the ASH-RhoGAP–like domain tested (20, 29), the recently discovered patient mutations N591K, L634P, P799L, and P801L abolish APPL1 binding while preserving clathrin binding. P799L mutation causes Dent disease. All mutants deficient for APPL1 binding also are deficient for HA-Ses1 and HA-Ses2 binding; detectable but strongly decreased Ses2 binding occurred for the N591K mutant, including all mutants previously tested negative for APPL1 binding (Fig. S2) (20, 29). Two mutations (F668V and A861T) preserve both APPL1 and Ses1/2 binding. Collectively, these results indicate that APPL1 and Ses proteins use a binding modality similar to that of the OCRL ASH-RhoGAP–like domain.