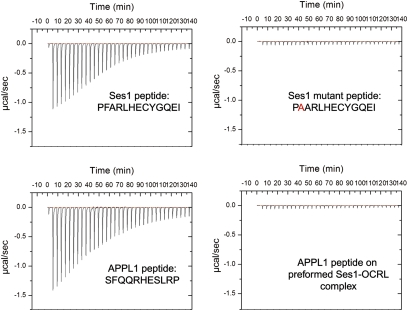
Fig. 5.
Direct binding of the Ses1 minimal 13 amino acid peptide to the OCRL ASH-RhoGAP–like domain. ITC was used to measure the affinity of the minimal OCRL-binding peptides of APPL1 (11 amino acids;12 ± 2 μM affinity) and Ses1 (13 amino acids; 0.7 ± 0.08 μM). As in the case of the APPL1 peptide (20), Ses1 binding critically depended on a conserved phenylalanine residue. The similarity between Ses- and APPL1-binding properties suggested a competitive mechanism for OCRL binding. Accordingly, a Ses peptide:OCRL ASH-RhoGAP complex saturated for Ses binding did not bind the APPL1 peptide.