Abstract
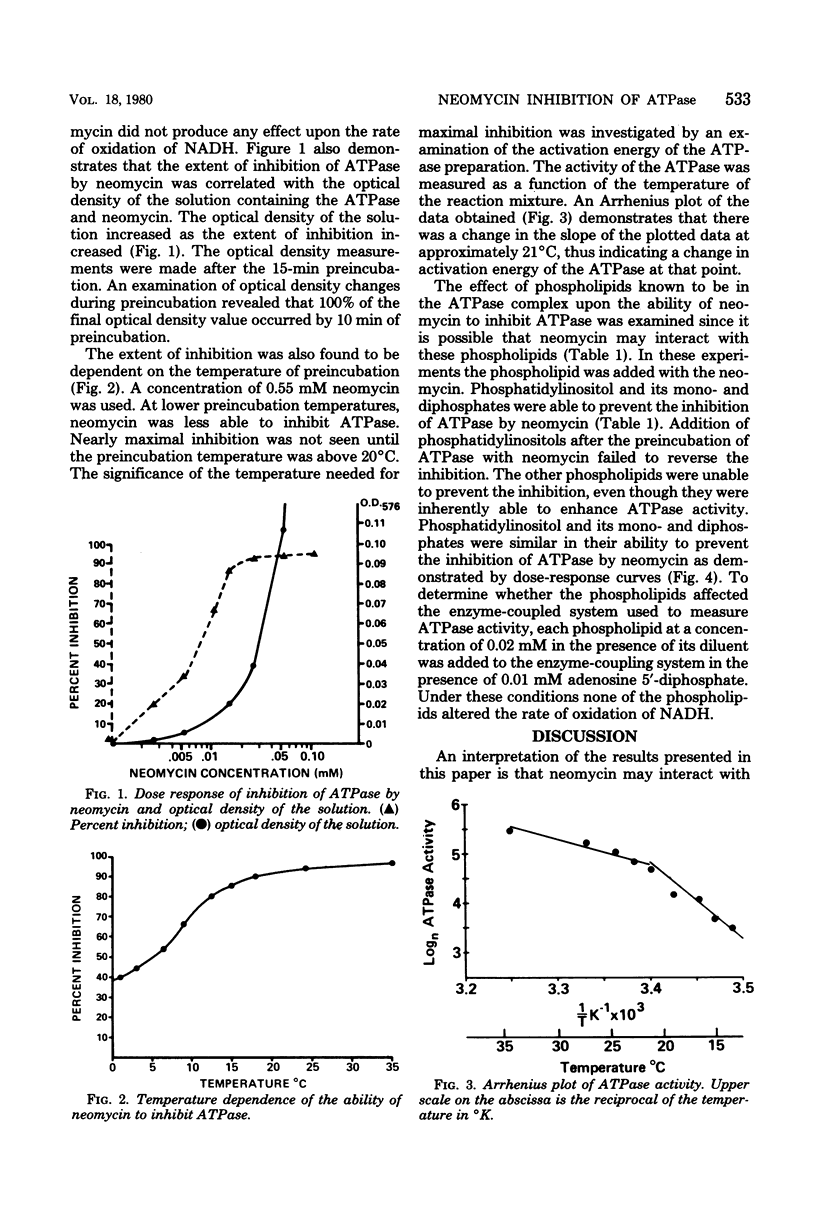
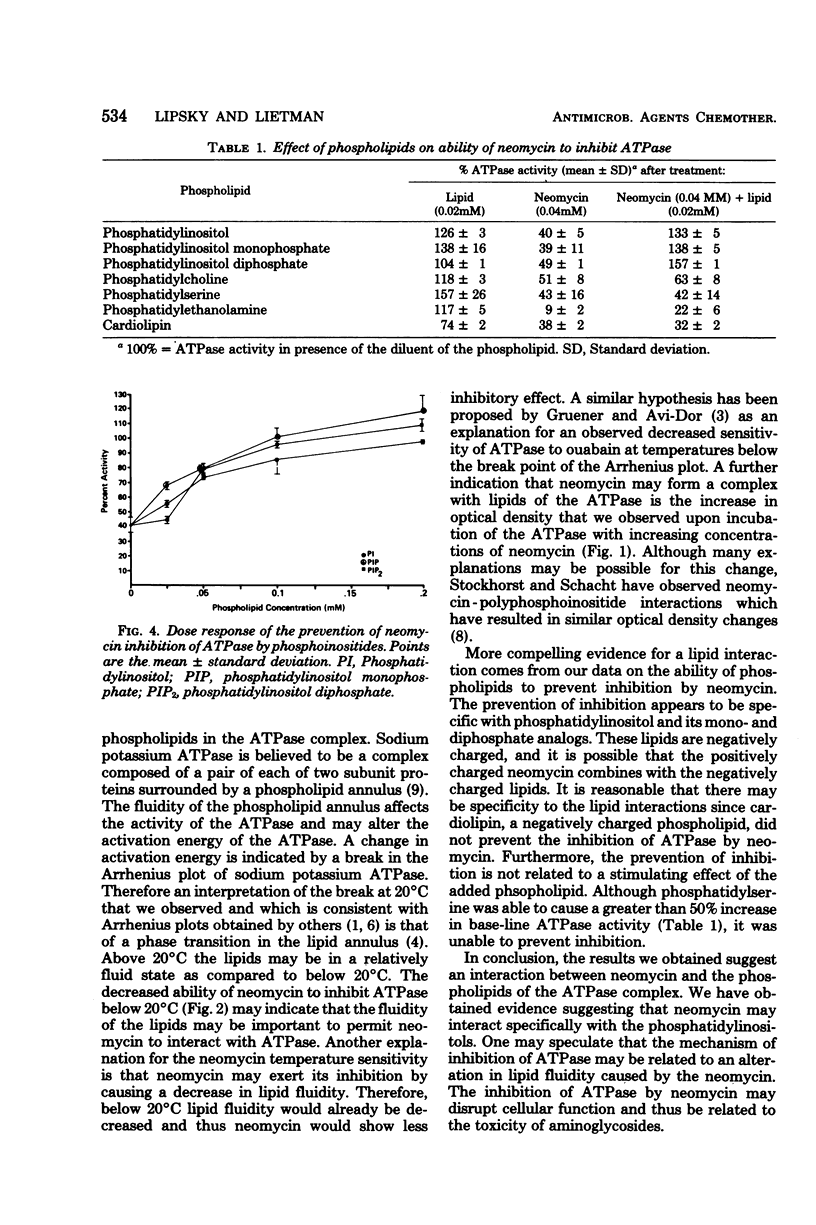
The inhibition of canine renal sodium potassium adenosine triphosphatase (ATPase) by neomycin was examined. Neomycin inhibited ATPase nearly maximally at 0.02 mM. The inhibition was temperature dependent with a decrease in inhibition occurring at temperatures below 21 degrees C, a temperature which corresponded to a change in activation energy of the ATPase as determined by Arrhenius plot. Preincubation of the ATPase with phosphoinositides was found to prevent the inhibition by neomycin. Other phospholipids were not found to prevent the inhibition. These results indicate a possible interaction between neomycin and the phosphoinositides of the ATPase complex.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Charnock J. S., Cook D. A., Casey R. The role of cations and other factors on the apparent energy of activation of (Na + + K + )-ATPase. Arch Biochem Biophys. 1971 Nov;147(1):323–329. doi: 10.1016/0003-9861(71)90340-7. [DOI] [PubMed] [Google Scholar]
- Dahl J. L., Hokin L. E. The sodium-potassium adenosinetriphosphatase. Annu Rev Biochem. 1974;43(0):327–356. doi: 10.1146/annurev.bi.43.070174.001551. [DOI] [PubMed] [Google Scholar]
- Gruener N., Avi-Dor Y. Temperature-dependence of activation and inhibition of rat-brain adenosine triphosphatase activated by sodium and potassium ions. Biochem J. 1966 Sep;100(3):762–767. doi: 10.1042/bj1000762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelberg H. K. Protein-liposome interactions and their relevance to the structure and function of cell membranes. Mol Cell Biochem. 1976 Feb 25;10(3):171–190. doi: 10.1007/BF01731688. [DOI] [PubMed] [Google Scholar]
- Orsulakova A., Stockhorst E., Schacht J. Effect of neomycin on phosphoinositide labelling and calcium binding in guinea-pig inner ear tissues in vivo and in vitro. J Neurochem. 1976 Feb;26(2):285–290. doi: 10.1111/j.1471-4159.1976.tb04478.x. [DOI] [PubMed] [Google Scholar]
- PULLMAN M. E., PENEFSKY H. S., DATTA A., RACKER E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. I. Purification and properties of soluble dinitrophenol-stimulated adenosine triphosphatase. J Biol Chem. 1960 Nov;235:3322–3329. [PubMed] [Google Scholar]
- Priestland R. N., Whittam R. The temperature dependence of activation by phosphatidylserine of the sodium pump adenosine triphosphatase. J Physiol. 1972 Jan;220(2):353–361. doi: 10.1113/jphysiol.1972.sp009711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockhorst E., Schacht J. Radioactive labeling of phospholipids and proteins by cochlear perfusion in the guinea pig and the effect of neomycin. Acta Otolaryngol. 1977 May-Jun;83(5-6):401–409. doi: 10.3109/00016487709128864. [DOI] [PubMed] [Google Scholar]
- Sweadner K. J., Goldin S. M. Active transport of sodium and potassium ions: mechanism, function, and regulation. N Engl J Med. 1980 Apr 3;302(14):777–783. doi: 10.1056/NEJM198004033021404. [DOI] [PubMed] [Google Scholar]


