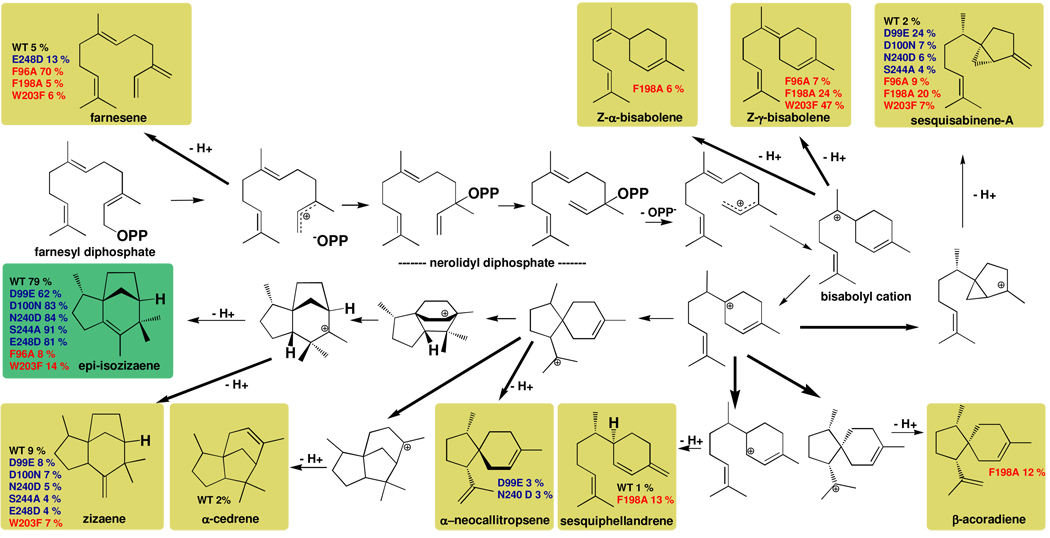
Figure 6.
The biosynthetic versatility of EIZS can be manipulated by site-directed mutagenesis, as illustrated for sesquiterpene products identified for wild-type (WT) and mutant cyclases. In general, more diverse sesquiterpene product arrays result from the substitution of aromatic residues defining the active site contour (red labels) than from substitution of residues that coordinate the Mg2+ ions required for catalysis (blue labels (14)). For example, F198A EIZS does not generate epi-isozizaene at all, but instead generates a mixture of sesquisabinene-A, Z-α-and Z-γ-bisabolenes, sesquiphellandrene, and β-acoradiene as its major cyclization products. Remolding the active site contour permits the generation of alternative products as long as they can be accommodated within the remolded template, as illustrated for β-acoradiene in Figure 5. It is interesting to note that many of these reactions have been examined in theoretical and computational studies (33).