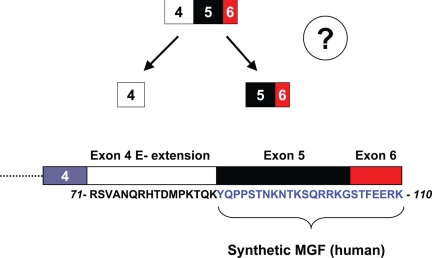
Figure 3.
Required step for derivation of MGF from the rest of the E-peptide. For MGF to exist as an endogenous 25-amino-acid peptide (24 amino acids in humans due to absence of one codon within exon 5; human sequence is shown), a processing step must occur that removes the 16-amino-acid E-extension from the peptide generated from exon 5 and exon 6 (see also Table 2); this step has not been observed in any system to date. Additionally, there is no identified sequence or motif within the intact 41-amino-acid E-peptide that would suggest this step occurs. For an endogenous peptide equivalent to the synthetic MGF to exist, proteolytic cleavage must occur that liberates those residues encoded by exon 5–6 (blue text) from those encoded by the exon 4 E-extension (black text). No furin or furin-like convertase, which are known to process pro-IGF-I, cleave scissile bonds on the carboxyl end of lysine or on the amino end of tyrosine.