Figure 5.
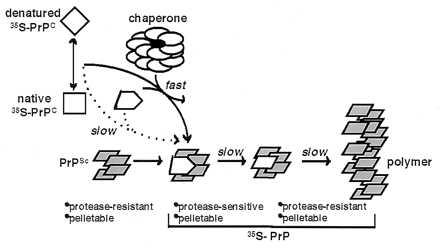
Model for chaperone-supervised PrP conversion. Conversion of [35S]PrPC to PrP-res in vitro requires pre-existing PrPSc (refs. 17–22, and this study). Without chaperone, conversion is slow and inefficient likely because [35S]PrP intermediates that productively associate with PrPSc are sparsely populated. The chaperone likely recognizes and binds near-native and nonnative intermediates derived from acid-treated [35S]PrPC, alters their conformation, and releases them in states that associate productively with PrPSc. Thereby, the chaperone facilitates the first step in conversion: specific binding of [35S]PrP to PrPSc. In this stage, [35S]PrP is pelletable, but remains protease-sensitive. A second slower step then follows, wherein PrPSc-bound [35S]PrP undergoes a second conformational transition to form PrP-res, the converted state with protease digestion properties strikingly similar to PrPSc.
