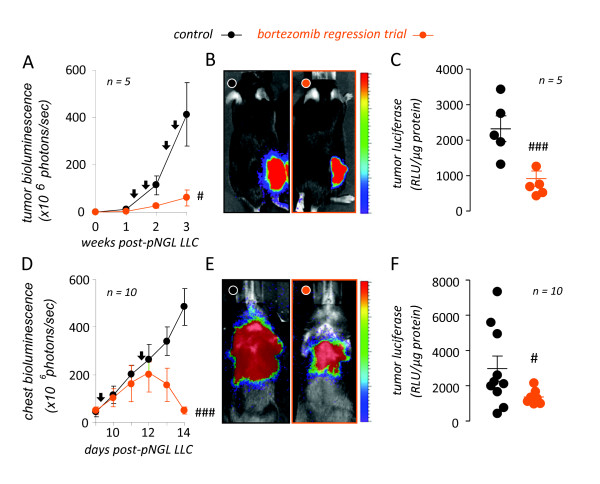
Figure 2.
Tailored bortezomib treatment inhibits tumor-specific nuclear factor (NF)-κB activation of subcutaneous and intrapleural lung adenocarcinoma. (A-C) NF-κB-targeted bortezomib treatment blocks NF-κB activation of subcutaneous adenocarcinoma in vivo. Wild-type C57BL/6 mice received 5 × 105 subcutaneous NF-κB-reporter (NF-κB. GFP.luc, pNGL) Lewis lung carcinoma (LLC) cells and were allowed one week for tumors to implant, where after they received bi-weekly intraperitoneal bortezomib (100 ng/g = 0.1 mg/kg) or PBS in a regression trial. Mice were serially imaged for bioluminescence after intravenous injection of 1 mg D-luciferin at the time-points indicated (A, B) and were sacrificed on day 21 for determination of NF-κB-dependent luciferase bioactivity of tumor tissue homogenates (C). Shown are time course of tumor-specific NF-κB activity in flank tumors (A), representative bioluminescence images at day 21 (B), and summary of luciferase assay data obtained at day 21 (C). (D-F) NF-κB-targeted bortezomib treatment blocks NF-κB activation of intrapleural adenocarcinoma in vivo. C57BL/6 mice received 1.5 × 105 intrapleural pNGL LLC cells and were allowed one week, where after they received bi-weekly bortezomib (100 ng/g = 0.1 mg/kg) or PBS. Mice were imaged for bioluminescence (D, E) and were sacrificed on day 14 for determination of NF-κB-dependent luciferase bioactivity of tumor tissue (F). Shown are time course of tumor-specific NF-κB activity in MPE-bearing mice (D), representative bioluminescence images at day 14 (E), and summary of luciferase assay data obtained at day 14 (F). n, sample size; RLU, relative light units. Dots, mean (left) or raw data points (right); lines in panels on the right, mean; bars, standard error of mean. # and ###: P < .05 and .001, respectively, compared with control.