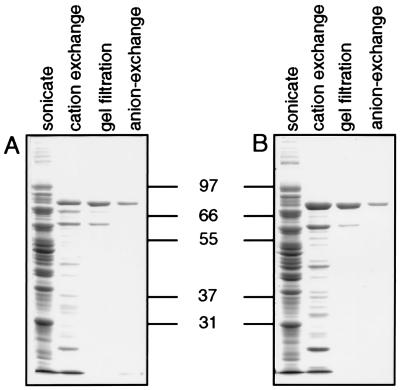
Figure 2.
Purification of functional recombinant PPPK-DHPS enzyme from P. falciparum in E. coli. Four liters of either D10-C or K1-C were grown and the PPPK-DHPS activity purified by sequential steps of cation-exchange, gel-filtration, and anion-exchange chromatography. Purification was monitored by enzyme activity and detection with anti-DHPS antibodies. Aliquots of D10-C (A) or K1-C (B) chromatography aliquots were separated by SDS/PAGE, and protein was detected by staining with Coomassie blue. Tracks are as indicated and are in both panels E. coli sonicate, pooled-positive cation-exchange, gel-filtration, and anion-exchange chromatography fractions.