Table 1.
Auxiliary Optimization Studiesa
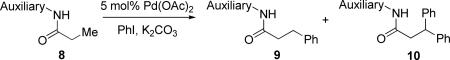 | ||||
|---|---|---|---|---|
| entry | amide | solvent, T | major product | NMR yield, % |
| 1 |
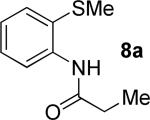
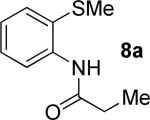
|
t-Amyl-OH, 90 °C |
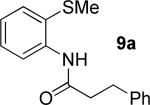
|
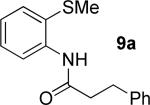
55% 9a 22% 10a |
| 2 |
|
t-Amyl-OH/H2O 4/1, 90 °C |
|
84% 9a 13% 10a |
| 3 |
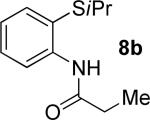
|
t-Amyl-OH/H2O 4/1, 90 °C |
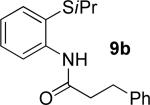
|
59% 9b 8% 10b |
| 4 |
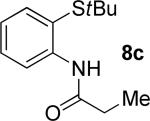
|
t-Amyl-OH/H2O 4/1, 90 °C |
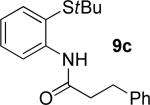
|
19% 9c |
| 5 |
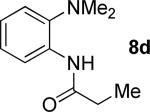
|
t-Amyl-OH/H2O 4/1, 110 °C |
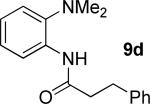
|
61% 9d 6% 10d |
| 6 |
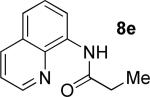
|
t-Amyl-OH/H2O 4/1, 110 °C |
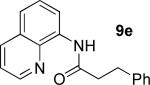
|
66% 9e 17% 10e |
| 7b |
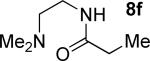
|
t-Amyl-OH/H2O 4/1, 90 °C |
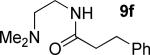
|
45% 9f |
| 8 |
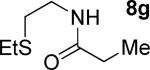
|
t-Amyl-OH/H2O 4/1, 90 °C |
|
10% 9g |
Amide (1 equiv), Pd(OAc)2 (0.05 equiv), base (2.5 equiv), and PhI (3 equiv). Yields are NMR yields against dichloroethane internal standard. See the Supporting Information for details.
Base used - K3PO4.
