Table 4.
Alkylation of C-H Bondsa
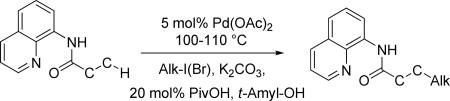 | ||||
|---|---|---|---|---|
| entry | amide | AlkI(Br) | major product | yield, % |
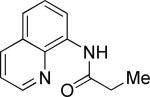
| 1 |
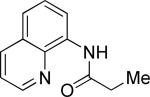
|
i-Butyl iodide |
|
58 |
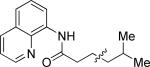
| 2 |
|
n-Octyl iodide |
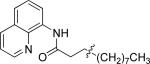
|
47 |
| 3 |
|
n-Propyl iodide |
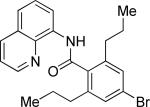
|
77 |
| 4 |
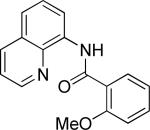
|
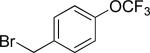
|
|
45 |
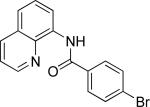
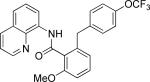
| 5 |
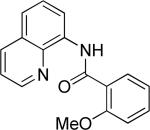
|
benzyl bromide |
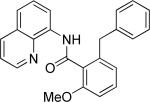
|
64 |
Amide (1 equiv), Pd(OAc)2 (0.05 equiv), base (2.5-3.5 equiv), pivalic acid (0.2 equiv), and RI or RBr (3-4 equiv) Yields are isolated yields. See the Supporting Information for details.
