Figure 2.
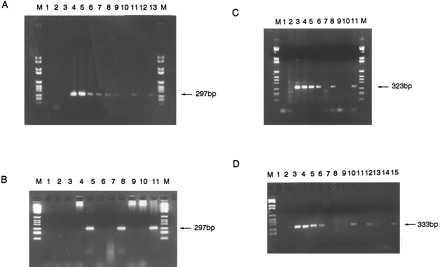
(A) PCR amplification of 297-bp-specific MLL-AF4 breakpoint region from diagnostic patient 1 leukemic DNA dilutions and two one-eighth segments of Guthrie spot. Lanes: M, DNA marker VI; 1, no DNA control; 2, 100 ng human placenta; 3, 100 ng patient 1 DNA with nonspecific primers; 4–10, patient 1 diagnostic DNA dilutions 100 ng–10 pg; 11, one-eighth segment patient 1 Guthrie; 12, “normal” Guthrie negative control; 13, one-eighth segment patient 1 Guthrie; M, DNA marker. (B) PCR on diagnostic DNA and third and fourth pieces of Guthrie spot from patient 1. Lanes: M, DNA marker VI; 1, no DNA control; 2, 100 ng human placenta negative control; 3 and 4, negative control one-eighth segments of normal Guthries 1 and 2; 5, third one-eighth segment of patient 1 Guthrie; 6 and 7, negative control normal Guthries 3 and 4; 8, fourth one-eighth segment of patient 1 Guthrie; 9 and 10, negative control normal Guthries 5 and 6; 11, patient 1 diagnostic DNA; M, DNA marker VI. (C) PCR amplification of specific 323-bp product of the AF4-MLL breakpoint region from patient 2 diagnostic DNA and two segments of Guthrie spot. Lanes: M, DNA marker VI; 1, no DNA control; 2, 100 ng patient 2 DNA with nonspecific primers; 3–6, patient 2 DNA 100 ng–0.1 ng; 7, 100 ng human placenta negative control; 8, one-eighth segment patient 2 Guthrie; 9 and 10, one-eighth segments of normal, negative control Guthries; 11, one-eighth segment of patient 2 Guthrie; M, DNA marker VI. (D) PCR amplification of 333-bp product specific to patient 3 AF4-MLL breakpoint region from diagnostic DNA and four one-eighth segments of Guthrie spot. Lanes: M, DNA marker VI; 1, no DNA control; 2, 100 ng human placenta negative control; 3–6, patient 3 diagnostic DNA 10 ng–10 pg; 7, patient 3 DNA with nonspecific primers; 8 and 9, one-eighth segments of negative control normal Guthries; 10–15, six one-eighth segments of patient 3 Guthrie spot.
