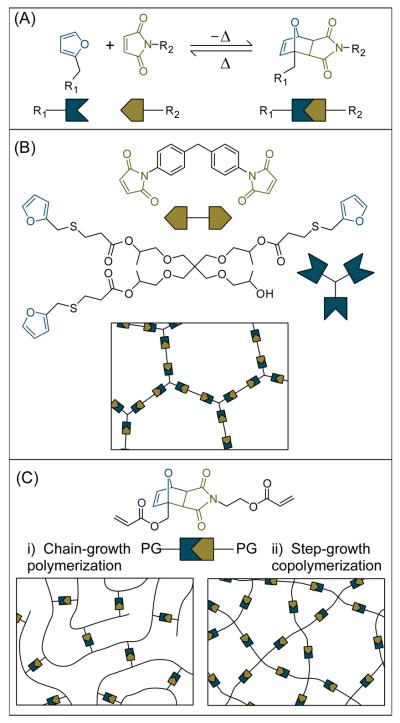
Figure 1. The thermoreversible Diels-Alder (DA) reaction between furan and maleimide (panel A) is used to demonstrate CAN fabricated by formation of the reversible linkage (panel B) or by polymerizable functionality flanking the reversible linkage (panel C).
In each of the panels, the reaction between maleimide and furan are represented as complementary geometric shapes. In panel A, the DA cycloaddition between furan (left) and maleimide (middle), which forms the bicyclic compound (right) at low temperatures, is shown. This model thermoreversible reaction undergoes the retro-Diels-Alder reaction at elevated temperatures. In panel B, the network is formed by bismaleimide and trisfuran monomers12 that undergo a step-growth polymerization via the reversible linkage. In panel C, the reversible CAN linkage is flanked by two polymerizable groups (PG) which, as an example, are given as acrylate functional groups. These acrylate functional groups can be either i) polymerized via a radical-mediated chain-growth mechanism13, 14 or ii) co-polymerized with a multifunctional thiol monomer (e.g., pentaerythritol-tetrakis-3-mercaptopropionate) via base-catalyzed Michael addition.15 The insets of B and C represent the formed network structure, which contains reversible linkages that enable reversible depolymerization. It should be noted that although a thermoreversible CAN was used as an example, this demonstration of polymerization types is equally applicable for photoreversible CANs.