Table 2.
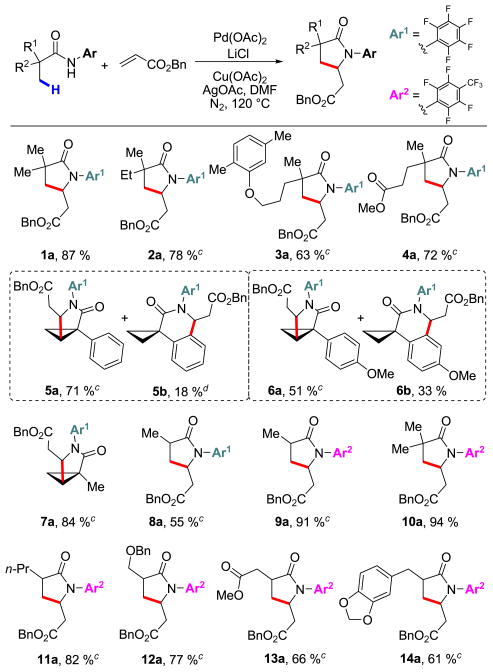 |
Conditions: 0.2 mmol of substrate, 0.1 mL of benzyl acrylate, 10 mol % Pd(OAc)2, 2.0 equiv of LiCl, 1.1 equiv of Cu(OAc)2, 1.1 equiv of AgOAc, 1 mL of DMF, 120 °C, N2, 12 h.
Isolated yield,.
Obtained as a mixture of inseparable cis/trans diastereomers (see Supporting Information). Optically inactive starting materials were used.
Yield determined by 1H NMR.
