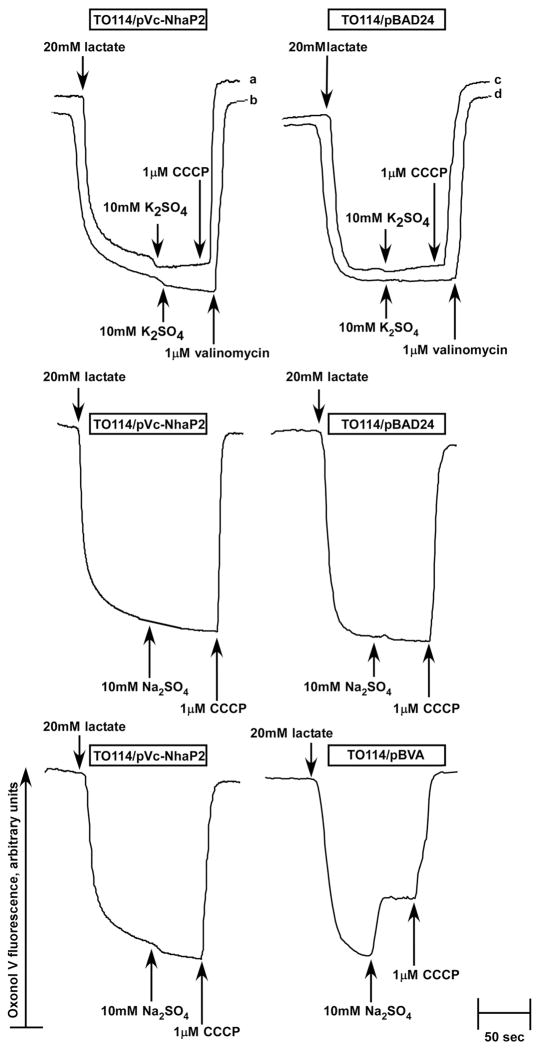
FIGURE 6.
Probing the stoichiometry of Vc-NhaP2. Inside-out membrane vesicles were isolated from TO114 transformants and assayed for ΔΨ at pH 7.5 in sorbitol-based medium devoid of K+ and Cl-. Diethanolamine at 20 mM was added to the experimental mixture 5 min prior to the addition of Oxonol V. At the indicated time, respiration-dependent formation of ΔΨ was initiated by the addition of 20 mM Tris-D-lactate. After steady-state ΔΨ was reached, cation/H+ antiport was initiated by the addition of 10 mM K2SO4 and 10 mM Na2SO4 (as indicated). The protonophore CCCP (traces marked “a”, “c”) or valinomycin in the presence of K+ (traces “b”, “d”) was added at the end of each measurement to collapse the generated ΔΨ for the control. The control experiment shown in the two lower panels compares side by side behavior of the electrogenic antiporter, Vc-NhaA (right lower panel, TO114/pBVA vesicles) and Vc-NhaP2 (left lower panel). Fluorescence of Oxonol V is shown in arbitrary units.