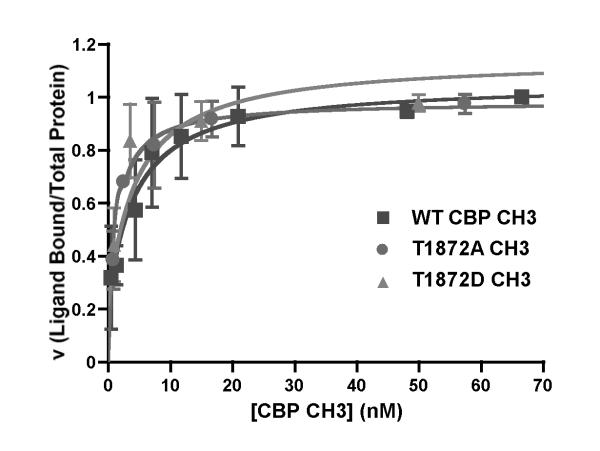
Fig. 6.
Equilibrium binding titrations of wildtype and mutant CBP CH3 with Akt1. Titration curves for WT CBP CH3 (squares), T1872A CH3 (circles), and T1872D CH3 (triangles) were derived from equation described in Materials and methods. Dissociation constants (KD) of WT CBP CH3, T1872A CH3, and T1872D CH3 for activated Akt1 are 4.30 ± 0.10 nM, 1.20 ± 0.01 nM, and 4.07 ± 0.67 nM, respectively.