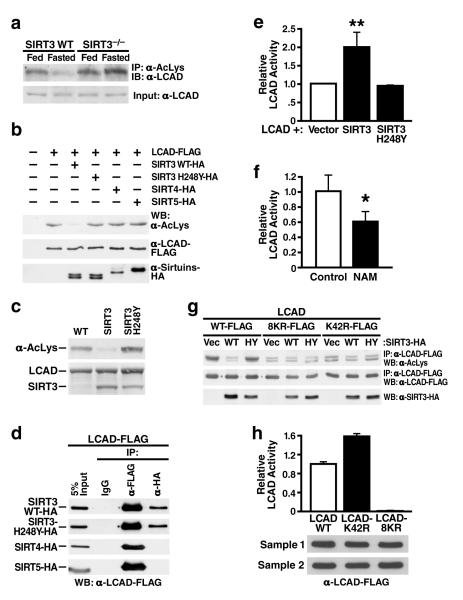
Figure 4. LCAD is hyperacetylated in SIRT3-/-mice, deacetylated by SIRT3 in vivo and in vitro, and displays increased enzymatic activity when deacetylated.
a. Liver extracts from wt and SIRT3-/- mice (fed or fasted) were immunoprecipitated with an anti-acetyllysine antiserum and analyzed with anti-LCAD antiserum; b. Expression vectors for wt SIRT3, SIRT3-H248Y (catalytically-inactive SIRT3 mutant), SIRT4, or SIRT5 were co-transfected with expression vectors for FLAG-tagged LCAD and the level of LCAD acetylation was assessed; c. Recombinant LCAD expressed in E. coli was incubated in vitro with recombinant SIRT3 or SIRT3-H248Y, and LCAD acetylation status was assessed; d. Expression vectors for wt SIRT3, SIRT3-H248Y, SIRT4 and SIRT5 (HA-tagged) were co-transfected with expression vectors for FLAG-tagged LCAD and assessed for interaction by co-immunoprecipitation; e. LCAD was expressed and purified with SIRT3 or SIRT3-H248Y and its enzymatic activity measured in vitro using 2, 6 dimethylheptanoyl-CoA as a substrate (n=4 independent assays); f. Recombinant LCAD was expressed in E. coli in the absence (Control) or presence of nicotinamide (NAM, 50 mM), purified and its enzymatic activity measured in vitro using 2, 6 dimethylheptanoyl-CoA as a substrate (n=4 independent assays); g. Expression vectors for wt LCAD, LCAD single acetylation point mutant (LCAD-K42R), or LCAD eight acetylation point mutant (LCAD-8KR) were co-transfected with expression vectors for wt SIRT3 or SIRT3-H248Y, and the level of acetylation was assessed; h. Wild-type LCAD, LCAD-K42R, or LCAD-8KR were expressed, and measured for enzymatic activity in vitro using 2, 6 dimethylheptanoyl-CoA as a substrate (n=5 measurement/sample, error bars represent data two independent protein purifications); *p<0.05, **p<0.01.