Abstract
Mitochondria accumulate at sites of intense metabolic activity within cells, but the adaptive value of this placement is not clear. In Drosophila, sesB encodes the ubiquitous isoform of adenine nucleotide translocase (ANT, the mitochondrial inner membrane ATP/ADP exchanger); null alleles are lethal, whereas hypomorphic alleles display sensitivity to a range of stressors. In the adult renal tubule, which is densely packed with mitochondria and hence enriched for sesB, both hypomorphic alleles and RNA interference knockdowns cause the mitochondria to lose their highly polarized distribution in the tissue and to become rounded. Basal cytoplasmic and mitochondrial calcium levels are both increased, and neuropeptide calcium response compromised, with concomitant defects in fluid secretion. The remaining mitochondria in sesB mutants are overactive and maintain depleted cellular ATP levels while generating higher levels of hydrogen peroxide than normal. When sesB expression is knocked down in just tubule principal cells, the survival of the whole organism upon oxidative stress is reduced, implying a limiting role for the tubule in homeostatic response to stressors. The physiological impacts of defective ANT expression are thus widespread and diverse.
Keywords: Drosophila melanogaster, Malpighian tubule, adenine nucleotide translocase, mitochondria
mitochondria are essential organelles for the function of all aerobic cells, as they produce ATP, buffer cytosolic calcium, and sequester apoptotic factors. Impairment of mitochondrial function, resulting in excitotoxic metabolic insufficiency, cell death, and oxidative damage, can contribute to severe tissue pathologies and diseases (36, 44). Mitochondria are also highly dynamic, and accumulate at sites with particularly high ATP consumption (3, 13, 17, 31, 37).
The mitochondrial adenine nucleotide translocase (ANT) is the most abundant protein of the inner mitochondrial membrane and a crucial component in the maintenance of cellular energy homeostasis, as well as in the formation of the mitochondrial permeability transition pore (2, 15). It catalyzes ADP/ATP exchange across the mitochondrial inner membrane (24) and thus has the potential to allow mitochondrial sensing of subcellular ATP:ADP poise. ANT is a target of oxidative damage, and functional inactivation has been associated with chronological age as well as physiological age (51). In mammals, the majority of species, including humans, possess three distinct genes for ANT (9, 25, 26, 28). Drosophila harbors two overlapping ANT genes, ANT1 (sesB), and ANT2, with 72% nucleotide identity with 78% amino acid sequence identity (52). Mutations affecting ANT1 in Drosophila were originally identified as a stress-sensitive strain of animals (sesB) that are conditionally paralytic in response to mechanical stress (18, 19). It was later discovered that sesB1 is an adult viable hypomorphic allele of the gene encoding ANT1 and that null alleles are lethal (52). Mutations have been suggested to cause a low rate of ATP pumping into the cytoplasm and cause defects in vesicle recycling (40).
The role of ANTs in specific tissues within the whole organism can be studied in Drosophila using the GAL4 > UAS system (6). In this study, we used targeted over- or underexpression of sesB to investigate its importance in mitochondrial function in the Malpighian (renal) tubule, a simple transporting epithelium (12) with an extraordinarily density of mitochondria (48), and thus highly enriched for ANT transcripts. The Drosophila tubule provides a genetically tractable, metabolically highly active model epithelium for which multiple functional readouts are available (12). Here, we aimed to characterize the in vivo role of the sesB (ANT1) gene in cytosolic and mitochondrial Ca2+ signaling. In parallel, we assessed changes in mitochondrial morphology and distribution, both in the sesB1 mutant and in flies in which RNA interference (RNAi) against sesB is expressed specifically in the Malpighian tubules, resulting in impairment of fluid secretion. Furthermore, manipulation of ANT1 in Malpighian tubule principal cells increased hydrogen peroxide (H2O2) production in both tubules and in isolated mitochondria and was sufficient to confer an organismal oxidative stress sensitive phenotype.
MATERIALS AND METHODS
Drosophila stocks and generation of transformants.
All strains were maintained on a standard Drosophila diet at 22°C, 55% humidity on a 12:12 h light-dark photoperiod. Wild-type flies were obtained from a Canton-S (CS) stock. The c42-GAL4 driver drives expression in tubule principal cells in the adult (7) and so is suitable for studies on acutely dissected adult tubules. The doubly homozygous c42-GAL4 > UAS-GFPmt (c42mtGFP) flies, generated by classical recombination, express the fluorescent GFP reporter in principal cell mitochondria. To assess in vivo calcium signals, doubly homozygous c42-GAL4 > UAS-apoaequorincyto (c42aeq) or c42-GAL4 > UAS-apoaequorinmt (c42mtaeq) flies were used, which express the apoaequorin luminescent calcium reporter in the cytosol or the mitochondria respectively, of the principal cells of the tubule main segment [upon which the diuretic neuropeptide Capa-1 acts (21, 42)]. Therefore, the doubly homozygous c42aeq, c42mtaeq flies were crossed with sesB mutant flies for cytosolic or mitochondrial calcium assays. For mitochondrial morphology and distribution in the principal cells of the tubule main segment, c42mtGFP flies were crossed with sesB mutant flies. The c710aeq flies express the apoaequorin transgene in the cytosol of the stellate cells of the tubule main segment. The doubly homozygous c710-GAL4 > UAS-apoaequorincyto (c710aeq) flies express the apoaequorin luminescent calcium reporter in the cytosol of the stellate cells of the tubule main segment (42). The ubiquitous Actin-GAL4 and hs-GAL4 lines were obtained from the Bloomington Stock Center (Bloomington, IN). c42-GAL4 also directs expression in a few other tissues in the adult fly (30). So to assess the impact of tubule-targeted sesB transgenes on the survival of whole flies under oxidative stress, we used our newly developed Urate Oxidase-GAL4 (UrO-GAL4) driver, based on the promoter region of a gene with expression utterly specific to principal cells of the main segment of third instar larval and adult tubules [expression only in the principal cells of the tubule main segment (46a)]. The use of the heat shock GAL4 driver (hs-GAL4), which drives the expression of the sesB RNAi transgene in all tubule cell types, enables us to use the diuretic neuropeptides Capa-1 (acting on principal cells) and Drosokinin (acting on stellate cells) sequentially in the same fluid transport assay to maximally stimulate the tubules.
As sesB is a recessive lethal locus, we used two classes of hypomorphic allele: heterozygotes for the sesB1 mutant (obtained from the Bloomington Stock Center, Bloomington, IN) and UAS-controlled RNAi alleles. To generate constructs for heritable RNAi of sesB gene, an inverted repeat of a 441-base pair fragment of sesB was generated by PCR using the primers 5′CTTGGCTGCTGATACTGGCAAGGGTGG-3′and 5′-AGCACGAAAGCGCCACCAGTTCCTCTG-3′ and cloned as a tail-tail inverted repeat flanking the white intron into the P-element vector pWIZ (27). The construct was injected into w1118 embryos by Bestgene, producing transgenic flies, in which hairpin ds-RNA could be expressed in cells of choice under control of the appropriate GAL4 driver lines (6). Validation of the sesB RNAi line was confirmed by quantitative (Q)-RT-PCR and Western blot analysis (Supplementary Figs. S1 and S21).
For overexpression studies, both open reading frames of the sesB gene (sesB-1 and sesB-2) were cloned using the Gateway system. The entry and destination vectors used were obtained from the Drosophila Gateway Vector collection (46). The primers listed were used in PCR for each of the sesB isoforms: 5′CACCATGGGCAAGGATTTCGATGCTGTT3′, 5′CAAGACCTTCTTGATCTCATCGTACAA3′ for sesB-1, and 5′CACCATGGGGAATATATCAGCATCCATA3′, 5′CAAGACCTTCTTGATCTCATCGTACAA3′ for sesB-2. Transgenic lines were generated using standard methods for P element-mediated germline transformation (BestGene). Independent transformants were isolated, and their chromosomal location determined according to standard genetic techniques. All transgenic lines were viable as homozygotes, thus facilitating crossing to produce flies in which the relevant reporter was targeted to cell-specific types using the appropriate GAL4 driver lines.
Calcium measurements using aequorin.
Luminometry experiments were carried out on live, intact tubules expressing a targeted transgene for either cytosolic or mitochondrially targeted apoaequorin as previously described (39, 48). For in vivo tubule experiments, flies of the following genotypes were used: c42aeq, c42mtaeq, c710aeq, UAS-sesB RNAi, and the resulting progeny from GAL4 > UAS crosses.
Immunocytochemistry.
The protocol used for immunohistochemistry was carried out as described previously (45) using mouse anti-GFP (1:1,000, Zymed), DAPI (1 μg/ml for 1 min, Sigma). An FITC-conjugated affinity-purified goat anti-mouse antibody (Jackson Immunologicals) was used in a dilution of 1:1,000 for visualization of the primary antiserum. The slides were viewed using a Zeiss 510 META confocal microscope.
Peptide antibody production.
Rabbit anti-peptide antibodies were raised against the sesB-2 epitope MGNISASITSQSKM (residues 1–14), sesB-1 and sesB-2 epitope RLAADTGKGGQREF (residues 155–158) by Genosphere Biotechnologies (Paris, France). Antisera were purified on a HiTrap NHS-activated HP column (Amersham Pharmacia Bio-tech, Buckinghamshire, UK) according to the manufacturer's instructions.
Renal fluid secretion assays.
Fluid transport assays were carried out as previously described (11) using live, intact tubules dissected from 7-day-old adults with the following genotypes: wild-type CS, sesB1 mutant, hs-GAL4 > UAS-sesB RNAi. Fluid droplets were collected every 10 min, and the volumes of fluid were calculated. Basal rates of fluid secretion were monitored for 30 min, whereupon Capa-1 and/or Drosokinin, endogenous Drosophila neuropeptides that stimulate Ca2+ signaling and fluid transport (21, 47), was added at 10−7 M, and the secretion rate was then recorded for a further 30 min.
Oxidative stress survival experiments.
Experiments were carried out as previously described (33). Briefly, 50 ml vials were prepared containing 2 ml of solid medium composed of 1.3% low melting agarose, 1% sucrose, and 1% H2O2. Three- to five-day-old flies were placed in each vial in groups of 25–30. Fly lines (mutant sesB1/Y and FM7a/Y, parental UO-GAL4, UAS-sesB-1, UAS-sesB-2, UAS-sesB RNAi; and progeny from GAL4 > UAS crosses) were maintained at 22°C, and numbers of surviving flies were counted at each time point. Survival curves were analyzed with the log-rank test (GraphPad Prism 4 software).
Organotypic imaging of mitochondria.
Live intact tubules were dissected from parental hs-GAL4, UAS-sesB RNAi, and progeny from GAL4 > UAS crosses allowed to adhere to glass-bottomed dishes coated with poly-l-lysine. Mitochondrial activity was assessed by treatment with the cationic and membrane-permeant mitochondrial dye, JC-1 (5 nM, Invitrogen), and imaged by confocal microscopy as previously described (48).
ATP assays.
ATP measurements were accomplished with a luciferin-based ATP Determination kit (Invitrogen). For each sample, 20 tubules were dissected and placed into 50 μl of Schneider's medium. Triplicate samples were generated for each genotype. All samples were sonicated, boiled for 10 min, and spun down at 5 000 g for 5 min at 4°C, and 25 μl of supernatant from each sample were used for the experiment. The assay was conducted using a Mithras LB 940 plate reader luminometer (Berthold technologies) according to manufacturer's instructions using ATP standards at final concentrations between 1 nM and 1 μM. Tubule samples were used at 1:9 (vol/vol) dilution in a final volume of 250 μl and assayed in duplicate. The ATP concentrations of tubule samples were calculated from the standard curve. Results are expressed as pmol ATP ± SE (n = 6).
Measurement of H2O2 generation from tubules and from isolated mitochondria.
For tubule assays, 10 pairs of rapidly dissected tubules from each genotype were added to 60 μl of 25 mM sodium phosphate buffer (pH 7.4), ruptured by brief sonication to release cellular contents, and spun down briefly at 5000 g at 4°C; 50 μl of the supernatant was used immediately in the Amplex Red assay (53).
Preparation of isolated mitochondria: Mitochondria from fly lines (UAS-sesB RNAi, hs-GAL4, and heat-shocked progeny from the cross) were isolated using the Pierce Mitochondria Isolation Kit for Tissue (Pierce Biotechnology, Rockford, IL). In brief, ∼100 adult flies were homogenized with 7–10 strokes of the homogenizer in a 0.3 mg/ml solution of trypsin in PBS on ice for 3 min. A quick refrigerated centrifugation was performed to pellet the sample and to remove the trypsin solution, and the pellet was then resuspended in PBS containing 4 mg/ml of BSA to quench the proteolytic activity of trypsin. The homogenate was treated with the kit reagents in the presence of Protease Inhibitor Cocktail (Sigma) and then subjected to a series of graded centrifugations. The mitochondrial pellet was suspended in mitochondria resuspension buffer [250 mM mannitol, 5 mM HEPES (pH 7.4), and 0.5 mM EDTA], and the protein content of the isolated mitochondria was determined by Bradford assay. Mitochondrial pellets (30 μg of protein) were used for the measurement of H2O2 production, described above.
Statistical analysis.
Data are presented as means ± SE. Significance of differences was assessed with Student's t-test (two-tailed) for unpaired samples, with significance taken as P < 0.05, marked graphically with an asterisk.
RESULTS AND DISCUSSION
ANT transcripts are tissue specifically expressed.
The Drosophila genes ANT1 (sesB) and ANT2 are duplicated in tandem and transcribed from a common promoter (52). Why are there two such similar genes? Previously ANT-lacZ transgenic flies were shown to express lacZ widely throughout the developmental stages (23); however, recent postgenomic resources allow a more refined view. FlyAtlas, a comprehensive microarray-based atlas of gene expression in multiple Drosophila tissues (8), reports distinct expression patterns for sesB and ANT2 genes (Table 1). SesB is ubiquitously expressed, though with depressed expression in the ovary and particularly testis. By contrast, ANT2 is virtually testis-specific.
Table 1.
Expression levels of sesB and ANT mRNA in larval and adult Drosophila tissues
| Tissue | sesB mRNA Signal | Enrichment | ANT mRNA Signal | Enrichment |
|---|---|---|---|---|
| Brain | 4,088 ± 85 | 1.00 | 32 ± 2 | 0.30 |
| Head | 4,886 ± 180 | 1.20 | 25 ± 2 | 0.20 |
| Eye | 6243 ± 86 | 1.48 | 35 ± 1 | 0.29 |
| TAG | 5,600 ± 177 | 1.30 | 32 ± 2 | 0.30 |
| Salivary gland | 9,963 ± 447 | 2.37 | 46 ± 5 | 0.39 |
| Crop | 5,047 ± 293 | 1.20 | 25 ± 2 | 0.20 |
| Midgut | 3,381 ± 87 | 0.80 | 65 ± 6 | 0.50 |
| Tubule | 5,904 ± 186 | 1.40 | 35 ± 3 | 0.30 |
| Hindgut | 5,264 ± 120 | 1.30 | 36 ± 2 | 0.30 |
| Heart | 5,486 ± 298 | 1.30 | 16 ± 0 | 0.14 |
| Fat body | 5,945 ± 874 | 1.41 | 29 ± 6 | 0.24 |
| Ovary | 2,743 ± 6 | 0.70 | 14 ± 0 | 0.10 |
| Testis | 467 ± 20 | 0.10 | 1,145 ± 86 | 9.40 |
| MAG | 3,570 ± 89 | 0.80 | 30 ± 2 | 0.30 |
| Virgin spermatheca | 5,595 ± 137 | 1.33 | 40 ± 3 | 0.33 |
| Mated spermatheca | 5,280 ± 285 | 1.25 | 44 ± 3 | 0.36 |
| Adult carcass | 7,528 ± 487 | 1.80 | 38 ± 7 | 0.30 |
| Whole fly | 4,210 ± 254 | 121 ± 5 |
Data were obtained from http://flyatlas.org (8). The signals represent mRNA signals globally normalized against all samples on all 4 chips for a tissue by Affymetrix GCOS software tissues (8). TAG, thoracicoabdominal ganglion; MAG, male accessory glands. Enrichment denotes enrichment of mRNA relative to whole fly.
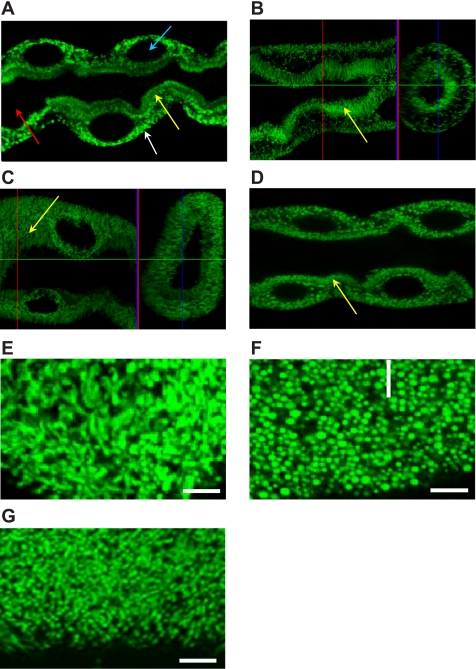
The Drosophila sesB gene encodes two isoforms: sesB-1 and sesB-2 (which encodes an extra 13 amino acids at the NH2 terminus). The Affymetrix probes used in FlyAtlas do not differentiate between these isoforms, but RT-PCR using primers designed to distinguish sesB-1 and sesB-2 transcripts confirmed that sesB-1 is ubiquitously expressed in the fly, while sesB-2 expression is not detected in tubules and seems to be almost specific to ovaries (Supplementary Fig. S3). These results suggest that ANT function is provided ubiquitously by sesB-1, though complemented with sesB-2 in ovary, while ANT2 is reserved for the functionally unique mitochondria of sperm. Within the tubule, confocal microscopy revealed mitochondrial localization of the sesB protein in principal cells of wild-type Malpighian tubule (Fig. 1A), which was identical to that seen with a UAS-mit-GFP reporter when driven in adult tubules using the c42-GAL4 driver (Fig. 1B).
Fig. 1.
Mitochondrial distribution in tubules from wild-type and sesB mutants. For orientation, arrows indicate apical mitochondria (yellow), basolateral mitochondria (white), principal cell nuclei (blue), and the tubule lumen (red) in A. A: immunocytochemistry using anti-sesB rabbit polyclonal antibody and anti-rabbit IgG-FITC conjugate reveal mitochondrial sesB protein localization in the main, fluid-transporting segment of the Malpighian tubule. B–F: expression of mitochondrial GFP reporter targeted to principal cells in adult tubules using the c42-GAL4 driver. B: control (c42mtGFP); C: SesB knockdown (c42mtGFP > sesB RNAi); D: SesB1 mutants (c42mtGFP > sesB1). In A–D, tubule diameter is taken as 30 μm. E–G: enlargement of basolateral plane, showing rod-like and branching normal mitochondria from c42mtGFP (E), compared with small globular mitochondria from c42mtGFP > sesB RNAi (F), and c42mtGFP > sesB1 tubules (G). In E–G, scale bar represents 1 μm.
To study the effect of sesB on fly development, we assayed for viability after sesB-1, sesB-2 overexpressor, and sesB RNAi knockdown in all tissues using the binary GAL4 > UAS system and an Actin-GAL4 strain (6). Phenotypic characterization of lethality revealed that ubiquitous over-expression of sesB-1 and sesB-2 were both 2nd instar larval lethal, while RNAi of sesB is lethal at pupal stage (data not shown). This confirms that normal levels of sesB are essential for development and viability of the fly.
SesB mutants reveal striking morphology and localization defects.
Imaging of mitochondria in tubules overexpressing sesB RNAi transgene reveals striking differences in the subcellular localization and shape of mitochondria (Fig. 1, C and D) compared with the control (Fig. 1B). These differences are shown clearly by transverse section of the tubule main segment. In c42mtGFP control flies (Fig. 1B), mitochondria are positioned adjacent to the basolateral membrane and packed in the microvillar membrane, while in the sesB RNAi tubules (Fig. 1C), mitochondria are distributed throughout the cell, with short and globular morphology. In sesB1 mutants (Fig. 1D) or tubules in which a sesB RNAi construct is driven (Fig. 1C), the pronounced apical and basal polarization of the mitochondria is lost, and they are distributed uniformly throughout the cytoplasm. In particular, the normally prominent threadlike brush border (Fig. 1B) is reduced and its mitochondrial density lower, in sesB mutants (Fig. 1, C and D). Furthermore, enlargement of basolateral plane shows that the mitochondria lose their normal threadlike appearance (Fig. 1E), and become short and globular (Fig. 1, F and G). This phenotype could be attributed to cytosolic calcium overload, which has been shown to alter mitochondrial morphology from elongated organelles into spherically shaped particles (4). Furthermore, alterations in mitochondrial morphology have been associated with mitochondrial metabolic state (14, 49) as well as apoptotic cell death (34).
SesB modulates both cellular and mitochondrial calcium levels.
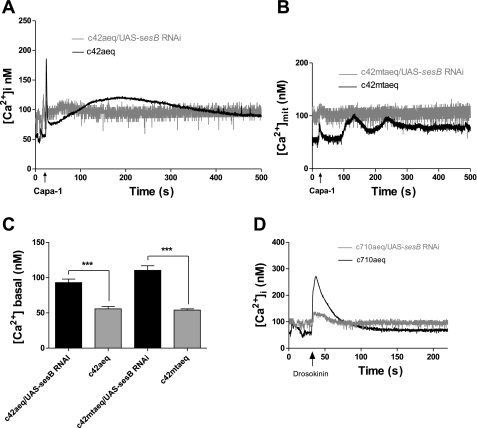
We previously argued that mitochondrial placement within the cell, and apposition to major sites of ATP consumption, was critical to the neuroendocrine control by CAPA peptides (48). In particular, the insertion of a mitochondrion into each apical microvillus provides a structural basis for the spatial and temporal filtering of the intracellular calcium signal received by the apical mitochondria. As sesB knockdown results in loss of this intimate association, the long-term phase of mitochondrial calcium signal should be lost. Accordingly, the impact of sesB on calcium signaling was determined in intact transgenic tubules using the bioluminescent calcium reporter aequorin (42). The Drosophila neurohormone Capa-1 acts upon the principal cells of tubules via an IP3-mediated pathway (38), producing a biphasic rise in cytosolic calcium (21) and eliciting calcium signals in the Golgi, peroxisomes (45), and mitochondria (48) via different calcium channels including TRP channels (10, 29). Transgenic fly lines homozygous for UAS-apoaequorincyto or UAS-apoaequorinmt, and the GAL4 enhancer trap insertion c42, which drives expression in adult tubule principal cells (22, 42), were crossed with UAS-sesB RNAi lines. Targeting sesB RNAi to tubule principal cells resulted in significantly increased basal cytoplasmic [Ca2+]i levels and abolished the Capa-1-induced calcium response (Fig. 2, A and C). Mitochondria are dynamic calcium stores (41) that track long-term changes in cytoplasmic calcium and are important in tubule function (10, 48); accordingly, sesB RNAi resulted in similarly increased basal [Ca2+]mit levels and a markedly inhibited mitochondrial calcium response to Capa-1 (Fig. 2, B and C).
Fig. 2.
SesB knockdowns increase basal calcium levels and blocks neuropeptide stimulation. A, B: expression of sesB RNA interference (RNAi) was targeted to principal cells in adult tubules expressing either the cytosolic (A) or mitochondrial (B) targeted calcium reporter, apoaequorin. Tubules were stimulated with the neuropeptide Capa-1 (10−7 M, arrowed), and the typical calcium response shown in black. Data are expressed as mean [Ca2+] (nM) ± SE; n > 10, P < 0.05, compared with wild-type tubules. C: bar graphs represent cytosolic and mitochondrial basal [Ca2+] from tubule principal cells overexpressing targeted sesB RNAi, average [Ca2+] (nM); n = 6, where ***P < 0.0001, Student's t-test. D: expression of sesB RNAi was targeted to stellate cells in adult tubules expressing the cytosolic calcium reporter. Tubules were stimulated with the neuropeptide Drosophila leucokinin (Drosokinin, 10−7 M, arrowed). Overexpression of sesB RNAi resulted in a significantly decreased Drosokinin calcium response.
The neuropeptide Drosophila leucokinin (Drosokinin) acts upon the stellate cells and elicits an increase in cytoplasmic Ca2+ via the IP3 pathway (38, 39). Targeting sesB RNAi to tubule stellate cells resulted in increased basal cytoplasmic [Ca2+]i levels and in significantly decreased Drosokinin-induced calcium response (Fig. 2D). Normal sesB expression is thus required both for cellular calcium homeostasis and the neuropeptide responses of both principal and stellate cells.
Tubule principal cell-specific expression of sesB modulates ATP levels.
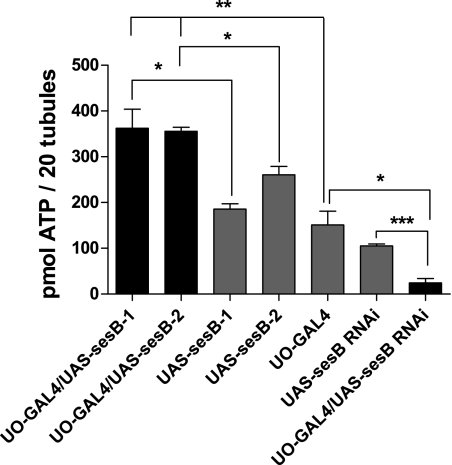
The importance of sesB in cellular energy homeostasis was demonstrated by assay of ATP levels in tubules using a luciferin-based ATP determination kit. Targeted overexpression of sesB-1 and sesB-2 in just tubule principal cells using the UO-GAL4 driver significantly increase the availability of ATP to the cell (Fig. 3A, black bars, P < 0.05 compared with parental lines). By contrast, RNAi against sesB expressed in the principal cells decreased tubule ATP levels significantly as predicted (Fig. 3B, black bar, P < 0.01 compared with parental lines). Although this reduction is not lethal, it might be expected to impact on tubule function.
Fig. 3.
Cellular ATP is modulated by sesB gene expression. Pools of 20 tubules were assayed for ATP content. Data are shown as means ± SE (n = 6). Significant difference *P < 0.05, **P < 0.01, and ***P < 0.001.
Effects of sesB gene on fluid transport.
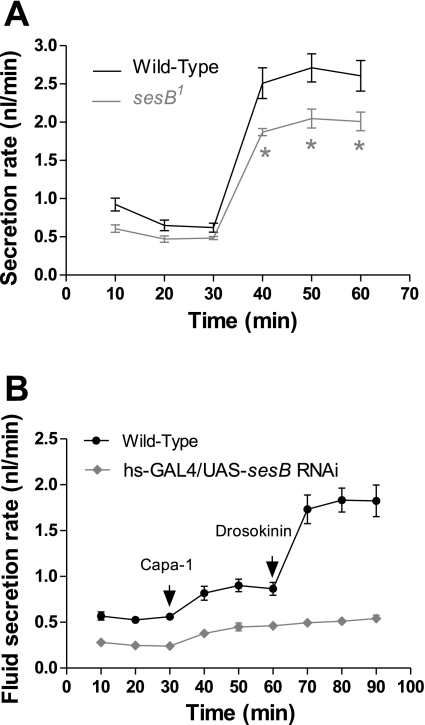
Capa-1 acts to stimulate fluid production through [Ca2+]mit by increasing ATP supply to the apical V-ATPase, which energizes secretion (48). With their reduced calcium response and lower levels of ATP, sesB mutants might be expected to show deficits in renal function. As predicted, Fig. 4A shows that viable hypomorphic alleles of sesB1 display a significantly reduced rate in fluid transport. Furthermore, phenotypic analysis of c42 > UAS-sesB RNAi Malpighian tubules reveal these transport fluid at reduced rates compared with wild type. Figure 4B shows that reducing sesB expression in principal cells significantly decreases basal rates of fluid transport, as well as the physiological response to the diuretic neuropeptides Capa-1 and Drosokinin. The compromised calcium signaling and ATP supply in sesB hypomorphs thus feed through to downstream functional properties.
Fig. 4.
Impact of sesB mutations on tubule function. A: fluid transport by Drosophila renal tubule is significantly decreased in hypomorphic sesB1 allele when the tubule is maximally stimulated by application of the neuropeptides Capa-1(10−7 M) and Drosokinin (10−7 M). *P < 0.05. B: tubules, in which UAS-sesB RNAi was driven by hs-GAL4, were stimulated at 30 min by the addition of Capa-1 and subsequently at 60 min with Drosokinin (arrows) cannot sustain high rates of stimulated fluid transport compared with wild type.
Does the mislocalization of mitochondria in sesB RNAi tubules cause the functional defects described or do the abnormalities in localization and function occurred in parallel? Malpighian tubules have intense metabolic demands, and the accepted paradigm is that mitochondria must be positioned properly within the cell to service regions of high ATP consumption. Only one study has shown that mitochondria move in and out of apical microvilli of insect tubules, Rhodnius prolixus (5), although our previous published work (48) showed mitochondrial activation in areas of high metabolic demand within the Drosophila Malpighian tubule. In this work, we demonstrate that the mitochondrial mislocalization and deficit of ATP supply is clearly manifested by fluid transport impairment in sesB mutants, suggesting that mislocalization contributes to functional physiological defects. However, it may also be that abnormalities in localization and function occur in parallel in the sesB mutants. For example, mitochondrial fission in cells is regulated by dynamin-related protein 1 (Drp-1). Dynamin activity appears to be regulated by the level of intracellular Ca2+ (16), and we have shown that calcium homeostasis is altered in sesB mutants, so it is possible that Ca2+ may directly activate Drp-1 and trigger mitochondrial fission.
Residual mitochondria in sesB mutants are overactive.
As well as altering placement, we observed reduced mitochondrial density in sesB mutants, suggesting that the remaining mitochondria might have to work harder to try to maintain ATP levels. Therefore, to investigate the potential effects on mitochondrial function by sesB RNAi transgene, the potential-sensitive dye JC-1 was used in live imaging experiments utilizing dissected tubules. A previous study has shown that, within renal principal cells, only basolaterally localized mitochondria are constitutively active, providing high levels of ATP for the function of basolateral ATPases (48). Figure 5B shows that targeted overexpression of sesB RNAi to tubule principal cells resulted in an increase in mitochondrial activation throughout the cell. It appears that, in sesB hypomorphs, the remaining mitochondria maintain tissue viability by increased activity, though at the potential risk of increased generation of reactive oxygen species (ROS).
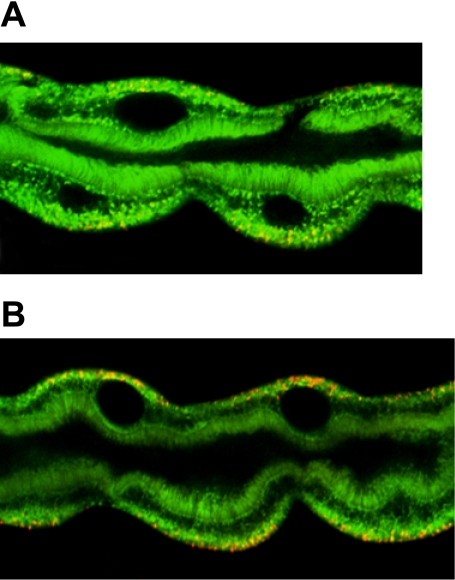
Fig. 5.
Mitochondria are overactive in sesB mutants. A, B: imaging of live tubules using mitochondrial potential-sensitive dyes. Activated mitochondria, in which the inner membrane potential is hyperpolarized, accumulate red fluorescence with JC-1 (48). A: mitochondrial activity in tubules from hs-GAL4 flies. B: note the increase in the red fluorescence compared with A in basal surface of tubule principal cells from hs-GAL4 > UAS-sesB RNAi flies.
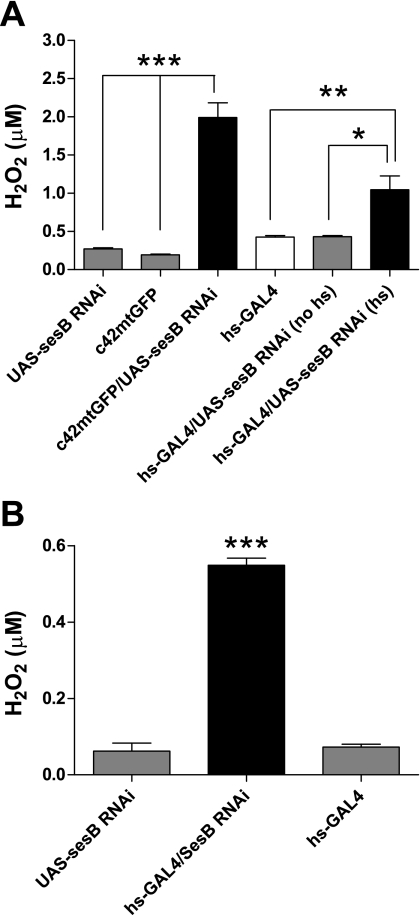
Does the low [ATP] generated by sesB RNAi tubules (Fig. 3) have an impact on H2O2 production? In mammalian mitochondria, low [ATP] can result in increased H2O2 production via different mechanisms at Complex 1, including a high NADH/NAD ratio (35). Indeed, H2O2 production increases sharply when SesB is knocked down with RNAi, both in whole tubules (Fig. 6A) and in mitochondria isolated from such tubules (Fig. 6B). However, given that current thinking suggests that in Drosophila mitochondria, O2·−/H2O2 production does not arise from NADH oxidation at Complex 1 (32), then this cannot provide a mechanistic link between the low ATP but high H2O2 observed in sesB RNAi tubules. An alternative explanation may lie with the role of glycerol 3-phosphate (G3P). Production of O2·−/H2O2 in Drosophila mitochondria has been shown to occur via action of glycerol 3-phosphate dehydrogenase (G3PDH), which converts G3P to dihydroxyacetone phosphate (DHAP) (32). The glycerol phosphate shuttle allows transport of electrons from cytosolic NADH to mitochondrial carriers of the oxidative phosphorylation pathway e.g., G3PDH. Assuming that this shuttle operates in flies, then if [ATP] is reduced, it is possible that glycolysis is increased, leading to increased [NADH], which in turn modulates activity of G3PDH, impacting on ubiquinone and also altering G3P/DHAP ratios. Data from Drosophila mitochondria show that production of O2·− arises from G3PDH activity and ubiquinone (32), on the cytoplasmic side of the inner membrane, suggesting that the glycerol phosphate shuttle may indeed result in increased O2·−/H2O2 production under conditions of low [ATP], as our data show.
Fig. 6.
Hydrogen peroxide (H2O2) production in tubules and in isolated mitochondria. A: detection of H2O2 in Malpighian tubules. Tubules from c42mtGFP > UAS-sesB RNAi and hs-GAL4 > UAS-sesB RNAi flies (heat-shocked) show increased H2O2 production compared with all tested genotypes. Data are shown as mean μM H2O2 ± SE (n = 6), where *P < 0.05, **P < 0.01, ***P < 0.001, Student's t-test. B: detection of H2O2 production in mitochondria. Mitochondria from heat-shocked hs-GAL4 > UAS-sesB RNAi flies show increased H2O2 production compared with parental lines. Data are shown as mean μM H2O2 ± SE (n = 3), where ***P < 0.001, Student's t-test.
Tubule knockdown of sesB sensitizes flies to oxidative stress.
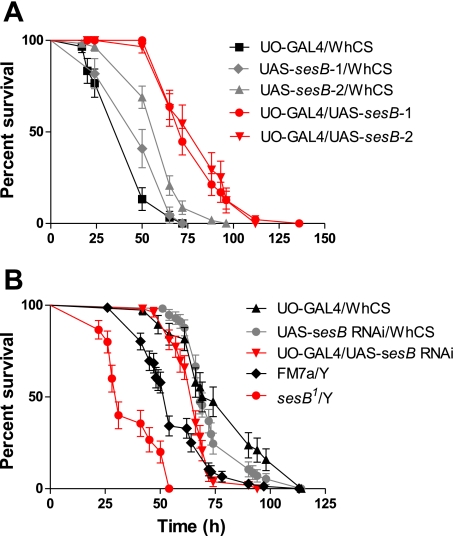
Previous work has shown that sesB mutation causes detectable phenotypes such as stress sensitivity or reduced viability. In sesB mutants, higher levels of endogenous ROS generation might compromise organismal response to oxidative stress, and help to explain the observed viability phenotypes. Targeted overexpression of sesB-1 and sesB-2 in just tubule principal cells using the UO-GAL4 driver significantly increases survival upon oxidative stress challenge by H2O2 (Fig. 7A, red line, P < 0.0001 compared with parental lines). By contrast, RNAi against sesB expressed in the principal cells significantly decreases survival in H2O2 medium (Fig. 7B, black line, P = 0.0014 compared with sesB RNAi/WhCS and P = 0.0004 compared UO-GAL4 > WhCS parental lines). Furthermore, Fig. 7B shows that the male viable hypomorphic allele sesB1 also displays a significantly reduced survival compared with FM7a siblings (red line, P < 0.0001 compared with parental line). Thus, a significant reduction in sesB expression in just tubule principal cells is deleterious for the whole fly, while overexpression of either sesB-1 or sesB-2 in tubule principal cells is sufficient to confer protection against H2O2-induced oxidative stress. This could be due to two reasons: cellular defense against oxidative stress could be dependent on cellular ATP levels, or the higher baseline ROS generation in sesB mutants may poise the organism to be more sensitive to additional external stress. Although we cannot rigorously distinguish these possibilities, the higher rates of ROS generation in overexpressors, and their protection against oxidative stress, argues for the former model.
Fig. 7.
Expression of sesBs exclusively in tubule principal cells modulates survival of the whole fly under oxidative stress. A, B: survival of flies after ingestion of H2O2. A: survival of flies in which sesB-1 and sesB-2 were targeted to tubule principal cells. B: survival of flies in which sesB RNAi was targeted to tubule principal cells and from male viable hypomorphic allele of sesB1. Outcrossed parental lines are shown in gray (UAS-sesB-1, UAS-sesB-2, and UAS-sesB RNAi) or black (UO-GAL4 > WhCS) and were generated to control for genetic background of the sesB transgenics stocks. Data are expressed as numbers of surviving flies up to 150 h ± SE, n > 30 flies per vial, repeated 3 times for each genotype.
Conclusion
Normal levels of ANT activity are necessary for mitochondrial function and thus viability. The tissue selectivity of ANT expression observed suggests that tuning of ANT function is critical for organismal survival. In humans, mutations in ANT1 (the heart and skeletal muscle isoform) demonstrate a range of symptoms from myopathy (1) to autosomal dominant progressive external ophthalmoplegia (20). Drosophila is a useful model system for study of mitochondrial defects (43), usually in the context of neural function (50). In Drosophila, sesB mutants were first obtained in a screen for mechanical stress sensitivity (52), and mutants have been shown to have an impact on synaptic transmission (40). This study focused on an epithelium in which sesB is highly enriched and for which a range of quantitative readouts are available. The range of defects observed - in morphology, placement, H2O2, and ATP production, secretion, and oxidative stress sensitivity - expand our understanding of the important roles played by the ANT transporter family.
GRANTS
This work was funded by grants from the UK Biotechnology and Biological Sciences Research Council.
DISCLOSURES
No conflicts of interest are declared by the authors.
Supplementary Material
ACKNOWLEDGMENTS
We thank Professor K. Beyenbach (Cornell) for helpful discussion.
Footnotes
The online version of this article contains supplemental material.
REFERENCES
- 1.Bakker HD, Scholte HR, Van den Bogert C, Ruitenbeek W, Jeneson JA, Wanders RJ, Abeling NG, Dorland B, Sengers RC, Van Gennip AH. Deficiency of the adenine nucleotide translocator in muscle of a patient with myopathy and lactic acidosis: a new mitochondrial defect. Pediatr Res 33: 412–417, 1993 [DOI] [PubMed] [Google Scholar]
- 2.Belzacq AS, Vieira HL, Kroemer G, Brenner C. The adenine nucleotide translocator in apoptosis. Biochimie 84: 167–176, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Boldogh IR, Pon LA. Mitochondria on the move. Trends Cell Biol 17: 502–510, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Boustany NN, Drezek R, Thakor NV. Calcium-induced alterations in mitochondrial morphology quantified in situ with optical scatter imaging. Biophys J 83: 1691–1700, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradley TJ, Satir P. 5-hydroxytryptamine-stimulated mitochondrial movement and microvillar growth in the lower malpighian tubule of the insect, Rhodnius prolixus. J Cell Sci 49: 139–161, 1981 [DOI] [PubMed] [Google Scholar]
- 6.Brand AH, Perrimon N. Targetted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118: 401–415, 1993 [DOI] [PubMed] [Google Scholar]
- 7.Broderick KE, Kean L, Dow JA, Pyne NJ, Davies SA. Ectopic expression of bovine type 5 phosphodiesterase confers a renal phenotype in Drosophila. J Biol Chem 279: 8159–8168, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Chintapalli VR, Wang J, Davies SA, Dow JAT. Using FlyAtlas to identify better Drosophila models of human disease. Nat Genet 39: 715–720, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Cozens AL, Runswick MJ, Walker JE. DNA sequences of two expressed nuclear genes for human mitochondrial ADP/ATP translocase. J Mol Biol 206: 261–280, 1989 [DOI] [PubMed] [Google Scholar]
- 10.Davies SA, Terhzaz S. Organellar calcium signalling mechanisms in Drosophila epithelial function. J Exp Biol 212: 387–400, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Dow JA, Maddrell SH, Gortz A, Skaer NJ, Brogan S, Kaiser K. The malpighian tubules of Drosophila melanogaster: a novel phenotype for studies of fluid secretion and its control. J Exp Biol 197: 421–428, 1994 [DOI] [PubMed] [Google Scholar]
- 12.Dow JAT, Davies SA. Integrative physiology and functional genomics of epithelial function in a genetic model organism. Physiol Rev 83: 687–729, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Frederick RL, Shaw JM. Moving mitochondria: establishing distribution of an essential organelle. Traffic 8: 1668–1675, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halestrap AP. Regulation of mitochondrial metabolism through changes in matrix volume. Biochem Soc Trans 22: 522–529, 1994 [DOI] [PubMed] [Google Scholar]
- 15.Halestrap AP, McStay GP, Clarke SJ. The permeability transition pore complex: another view. Biochimie 84: 153–166, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Hinshaw JE. Dynamin and its role in membrane fission. Annu Rev Cell Dev Biol 16: 483–519, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hollenbeck PJ, Saxton WM. The axonal transport of mitochondria. J Cell Sci 118: 5411–5419, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Homyk T. Behavioral mutants of Drosophila melanogaster. II. Behavioral analysis and focus mapping. Genetics 87: 105–128, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Homyk T, Sheppard DE. Behavioral mutants of Drosophila melanogaster. I. Isolation and mapping of mutations which decrease flight ability. Genetics 87: 95–104, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaukonen J, Juselius JK, Tiranti V, Kyttala A, Zeviani M, Comi GP, Keranen S, Peltonen L, Suomalainen A. Role of adenine nucleotide translocator 1 in mtDNA maintenance. Science 289: 782–785, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Kean L, Cazenave W, Costes L, Broderick KE, Graham S, Pollock VP, Davies SA, Veenstra JA, Dow JA. Two nitridergic peptides are encoded by the gene capability in Drosophila melanogaster. Am J Physiol Regul Integr Comp Physiol 282: R1297–R1307, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Kerr M, Davies SA, Dow JA. Cell-specific manipulation of second messengers; a toolbox for integrative physiology in Drosophila. Curr Biol 14: 1468–1474, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Kim YS, Shin MJ, Yang DJ, Yamaguchi M, Park SY, Yoo MA. Transcriptional regulation of the Drosophila ANT gene by the DRE/DREF system. Genes Cells 12: 569–579, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Klingenberg M. Mitochondrial carrier family: ADP/ATP carrier as a carrier paradigm. Soc Gen Physiol Ser 48: 201–212, 1993 [PubMed] [Google Scholar]
- 25.Ku DH, Kagan J, Chen ST, Chang CD, Baserga R, Wurzel J. The human fibroblast adenine nucleotide translocator gene. Molecular cloning and sequence. J Biol Chem 265: 16060–16063, 1990 [PubMed] [Google Scholar]
- 26.Kuan J, Saier MH., Jr The mitochondrial carrier family of transport proteins: structural, functional, and evolutionary relationships. Crit Rev Biochem Mol Biol 28: 209–233, 1993 [DOI] [PubMed] [Google Scholar]
- 27.Lee YS, Carthew RW. Making a better RNAi vector for Drosophila: use of intron spacers. Methods 30: 322–329, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Li K, Warner CK, Hodge JA, Minoshima S, Kudoh J, Fukuyama R, Maekawa M, Shimizu Y, Shimizu N, Wallace DC. A human muscle adenine nucleotide translocator gene has four exons, is located on chromosome 4, and is differentially expressed. J Biol Chem 264: 13998–14004, 1989 [PubMed] [Google Scholar]
- 29.MacPherson MR, Pollock VP, Kean L, Southall TD, Giannakou ME, Broderick KE, Dow JA, Hardie RC, Davies SA. Transient receptor potential-like channels are essential for calcium signaling and fluid transport in a Drosophila epithelium. Genetics 169: 1541–1552, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGettigan J, McLennan RK, Broderick KE, Kean L, Allan AK, Cabrero P, Regulski MR, Pollock VP, Gould GW, Davies SA, Dow JA. Insect renal tubules constitute a cell-autonomous immune system that protects the organism against bacterial infection. Insect Biochem Mol Biol 35: 741–754, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Minin AA, Kulik AV, Gyoeva FK, Li Y, Goshima G, Gelfand VI. Regulation of mitochondria distribution by RhoA and formins. J Cell Sci 119: 659–670, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Miwa S, St-Pierre J, Partridge L, Brand MD. Superoxide and hydrogen peroxide production by Drosophila mitochondria. Free Radic Biol Med 35: 938–948, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Monnier V, Girardot F, Audin W, Tricoire H. Control of oxidative stress resistance by IP3 kinase in Drosophila melanogaster. Free Radic Biol Med 33: 1250–1259, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Mootha VK, Wei MC, Buttle KF, Scorrano L, Panoutsakopoulou V, Mannella CA, Korsmeyer SJ. A reversible component of mitochondrial respiratory dysfunction in apoptosis can be rescued by exogenous cytochrome c. EMBO J 20: 661–671, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J 417: 1–13, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palladino MJ, Hadley TJ, Ganetzky B. Temperature-sensitive paralytic mutants are enriched for those causing neurodegeneration in Drosophila. Genetics 161: 1197–1208, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pilling AD, Horiuchi D, Lively CM, Saxton WM. Kinesin-1 and Dynein are the primary motors for fast transport of mitochondria in Drosophila motor axons. Mol Biol Cell 17: 2057–2068, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pollock VP, Radford JC, Pyne S, Hasan G, Dow JA, Davies SA. NorpA and itpr mutants reveal roles for phospholipase C and inositol (1,4,5)-trisphosphate receptor in Drosophila melanogaster renal function. J Exp Biol 206: 901–911, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Radford JC, Davies SA, Dow JA. Systematic G-protein-coupled receptor analysis in Drosophila melanogaster identifies a leucokinin receptor with novel roles. J Biol Chem 277: 38810–38817, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Rikhy R, Ramaswami M, Krishnan KS. A temperature-sensitive allele of Drosophila sesB reveals acute functions for the mitochondrial adenine nucleotide translocase in synaptic transmission and dynamin regulation. Genetics 165: 1243–1253, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Romagnoli A, Aguiari P, De Stefani D, Leo S, Marchi S, Rimessi A, Zecchini E, Pinton P, Rizzuto R. Endoplasmic reticulum/mitochondria calcium cross-talk. Novartis Found Symp 287: 122–131; discussion–131–129., 2007 [PubMed] [Google Scholar]
- 42.Rosay P, Davies SA, Yu Y, Sozen A, Kaiser K, Dow JA. Cell-type specific calcium signalling in a Drosophila epithelium. J Cell Sci 110: 1683–1692, 1997 [DOI] [PubMed] [Google Scholar]
- 43.Sanchez-Martinez A, Luo N, Clemente P, Adan C, Hernandez-Sierra R, Ochoa P, Fernandez-Moreno MA, Kaguni LS, Garesse R. Modeling human mitochondrial diseases in flies. Biochim Biophys Acta 1757: 1190–1198, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scharfe C, Lu HH, Neuenburg JK, Allen EA, Li GC, Klopstock T, Cowan TM, Enns GM, Davis RW. Mapping gene associations in human mitochondria using clinical disease phenotypes. PLoS Comput Biol 5: e1000374, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Southall TD, Terhzaz S, Cabrero P, Chintapalli VR, Evans JM, Dow JA, Davies SA. Novel subcellular locations and functions for secretory pathway Ca2+/Mn2+-ATPases. Physiol Genomics 26: 35–45, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Swanson CI, Hinrichs T, Johnson LA, Zhao Y, Barolo S. A directional recombination cloning system for restriction- and ligation-free construction of GFP, DsRed, and lacZ transgenic Drosophila reporters. Gene 408: 180–186, 2008 [DOI] [PubMed] [Google Scholar]
- 46a.Terhzaz S, Finlayson AJ, Stirrat L, Yang J, Tricoire H, Woods DJ, Dow JA, Davies SA. Cell-specific inositol 1,4,5 trisphosphate 3-kinase mediates epithelial cell apoptosis in response to oxidative stress in Drosophila. Cell Signal 2010January11[Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 47.Terhzaz S, O'Connell FC, Pollock VP, Kean L, Davies SA, Veenstra JA, Dow JA. Isolation and characterization of a leucokinin-like peptide of Drosophila melanogaster. J Exp Biol 202: 3667–3676, 1999 [DOI] [PubMed] [Google Scholar]
- 48.Terhzaz S, Southall TD, Lilley KS, Kean L, Allan AK, Davies SA, Dow JA. Differential gel electrophoresis and transgenic mitochondrial calcium reporters demonstrate spatiotemporal filtering in calcium control of mitochondria. J Biol Chem 281: 18849–18858, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Territo PR, French SA, Dunleavy MC, Evans FJ, Balaban RS. Calcium activation of heart mitochondrial oxidative phosphorylation: rapid kinetics of mVO2, NADH, AND light scattering. J Biol Chem 276: 2586–2599, 2001 [DOI] [PubMed] [Google Scholar]
- 50.Trotta N, Rodesch CK, Fergestad T, Broadie K. Cellular bases of activity-dependent paralysis in Drosophila stress-sensitive mutants. J Neurobiol 60: 328–347, 2004 [DOI] [PubMed] [Google Scholar]
- 51.Yan LJ, Sohal RS. Mitochondrial adenine nucleotide translocase is modified oxidatively during aging. Proc Natl Acad Sci USA 95: 12896–12901, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang YQ, Roote J, Brogna S, Davis AW, Barbash DA, Nash D, Ashburner M. stress sensitive B encodes an adenine nucleotide translocase in Drosophila melanogaster. Genetics 153: 891–903, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou M, Diwu Z, Panchuk-Voloshina N, Haugland RP. A stable nonfluorescent derivative of resorufin for the fluorometric determination of trace hydrogen peroxide: applications in detecting the activity of phagocyte NADPH oxidase and other oxidases. Anal Biochem 253: 162–168, 1997 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.