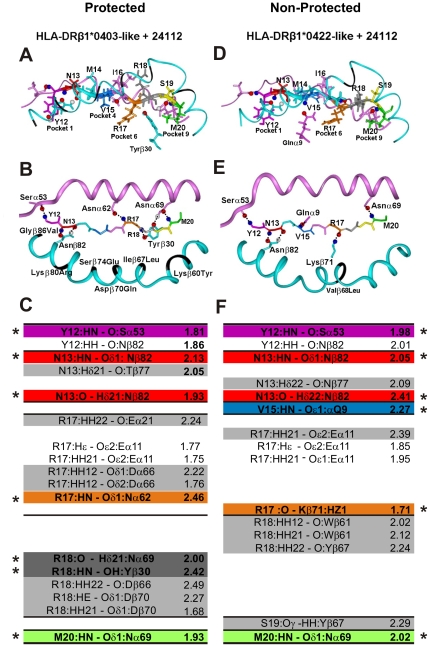
Figure 3. Interatomic interactions of peptide 24112 with HLA-DRβ1*04 molecules.
Interaction of peptide 24112 with the HLA-DRβ1*0403-like of protected Aotus 191: (A) frontal view, (B) top view. Interaction of peptide 24112 with the HLA-DRβ1*0422-like complex of the non-protected Aotus 148: (D) frontal view, (E) top view. In the frontal view panels A and D, the orientation of 24112 residues' lateral-chains (represented as sticks) and their positions inside MHCII molecules are shown according to the following color code: Y12 (fuchsia in P1), N13 (red in P2), M14 (pale blue in P3), V15 (dark blue in P4), I16 (pink in P5), R17 (orange in P6), R18 (dark gray in P7), S19 (yellow in P8) and M20 (green in P9). Top view panels B and E display the H bonds (shown as doted lines) established between backbone atoms of peptide 24112 (represented as sticks) and side-chain atoms of residues from the MHCII α and β chains (depicted as pink and blue ribbons, respectively) in protected (group B monkeys) as well as non-protected (group E monkeys). The nitrogen and oxygen atoms are shown as blue and red balls, respectively. Black segments in the β-chain correspond to the residues that were modified according to the MHCII sequence of Ao191 (HLA-DRβ1*0403) and Ao148 (HLA-DRβ1*0422). (C and F) H bonds and vdW interactions, measured in Amstrongs (Å), between 24112 with HLA-DRβ1*0403 and HLA-DRβ1*0422 molecules. Interactions involving different atoms are highlighted in pale gray, while interactions involving common residues are not shadowed. The color code for those residues establishing such H bonds is the same used in Figure 1.