Abstract
While the causes of multiple sclerosis (MS) are unknown, there is strong evidence that infection with Epstein–Barr virus (EBV) is an important factor. In this review, we discuss the epidemiological evidence and argue for a causal role of EBV in MS aetiology. One of the most striking and consistent observations is that MS is extremely rare among EBV-negative individuals. Further, the timing of EBV infection appears to be critical, with individuals who are infected during adolescence and young adulthood, when the infection is more likely to manifest as mononucleosis, having a two- to threefold greater risk of MS compared to individuals infected in early life. These observations challenge the hygiene hypothesis which states that being in a high hygiene environment in early life increases future risk of MS – if this general formulation were true, EBV-negative individuals would be expected to have an increased risk of MS. Additional support for the causal role of EBV comes from longitudinal, prospective studies which show remarkable consistency, in that antibodies against EBV are elevated prior to MS onset. However, while infection with EBV is consistent with many observations of MS epidemiology, there are some that remain unexplained, suggesting that other factors are also involved in determining risk.
Keywords: EBV, epidemiology, aetiology, infection, multiple sclerosis
Evidence of environmental factors in multiple sclerosis (MS)
MS is a relatively common disease in young adults in Europe, the United States, Canada, New Zealand and parts of Australia. In these high-risk populations, the lifetime risk of MS is about one in 200 for women and somewhat lower for men [1,2]. MS is rare in Asia and, in all continents, it is rare in the tropics and subtropics. Within regions of temperate climate, MS incidence and prevalence increase with latitude, both north and south of the equator [3,4].
Although genes contribute to the geographical distribution of MS, studies in migrants provide compelling evidence of strong environmental factors [5].
The incidence of MS in migrants tends to be intermediate between that of their birthplace and that of their final residence, and close to the latter when migration occurs in childhood [5]. Most convincing is the landmark investigation by Kurtzke et al. [6], demonstrating a reduction in risk among individuals who were born in the northern United States and entered military service in the South (Fig. 1).
Fig. 1.
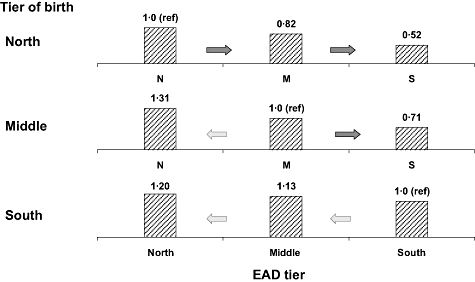
Relative risk of multiple sclerosis (MS) for white male veterans of World War II or the Korean conflict by tier of residence at birth and at entry into active duty (EAD) (coterminous United States only). Data from [6]. Reprinted with permission from Thieme Medical Publishers, Inc., Ascherio and Munger, Semin Neurol 2008; 28:17–28. P for no change in birthplace risk: all = 0·0003; north = 0·003; middle = 0·002; south = 0·57.
Infection as possible explanation of geographical variations and migrant data
Overall, the geographical distribution of MS and migrant data implicate environmental factors in childhood, and possibly during adult life, as strong determinants of MS risk, but do not identify the nature of these factors. Infections could play a role [7], as discussed below.
More than 40 years ago, Poskanzer proposed that MS could be caused by an infectious agent that is harmless and confers protective immunity when acquired in early childhood, but pathogenic when acquired later in life [8]. Thus, paradoxically, MS would be more common where its putative infectious cause is less prevalent and exposure is delayed. This hypothesis has often been called the ‘poliomyelitis’ hypothesis, because a late age at infection with the poliomyelitis virus was believed to be an important risk factor for the development of paralysis [8]. The poliomyelitis hypothesis evolved into a more general ‘hygiene hypothesis’[9], first proposed by Leibowitz following the observation of a positive correlation between degree of sanitation and MS incidence in Israel [10]. According to this hypothesis, exposure to infections early in life is protective against MS, as in the poliomyelitis model, but there is not a specific microbe that could be identified as the cause of MS [11–13]– rather, MS is an autoimmune reaction that can be triggered by multiple microorganisms in genetically susceptible individuals, and risk of clinical disease increases with age at infection [12,13]. The hygiene hypothesis could explain several features of MS epidemiology, including the latitude gradient, the apparent protection from MS of individuals born in low-risk areas who migrate to high-risk areas, the higher rates among individuals of higher education and income [14–16], the trend towards a later age at infection with childhood viruses in MS cases than controls [17], the lower risk of MS among individuals exposed to infant siblings in early life [18] and possibly the attenuation of the latitude gradient within the United States [2,19], which could be ascribed to improved hygienic conditions and thus lower incidence of childhood infections in the south.
Limitations of the hygiene hypothesis
In its general formulation, the hygiene hypothesis leads to the prediction that individuals who are not infected with the Epstein–Barr virus (EBV) should have a high risk of MS. This is because EBV infection in early childhood is associated strongly with hygienic conditions. Virtually all children are EBV seropositive at 4–6 years of age in developing countries, whereas in most industrialized countries 50% or more are still uninfected at these ages [20]. Thus, the lack of EBV antibodies in young adults is a strong marker of a hygienic upbringing, and as such should confer an increased MS risk. However, risk of MS among EBV negative individuals is extremely low (Fig. 2). This finding has been confirmed recently in studies of paediatric MS (Fig. 3) [22–24].
Fig. 2.
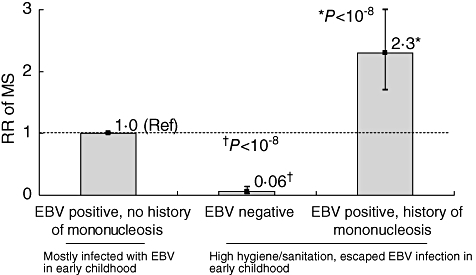
Relative risk (RR) of developing multiple sclerosis (MS) according to Epstein–Barr virus (EBV) infection and history of mononucleosis. Bars represent the 95% confidence intervals of the RR estimates. Data from [7,21] Reprinted with permission from Thieme Medical Publishers, Inc., Ascherio and Munger, Semin Neurol 2008; 28:17–28.
Fig. 3.
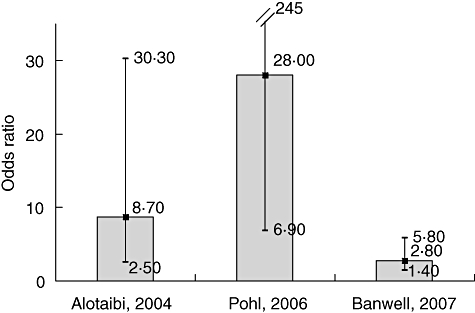
Odds ratio of paediatric multiple sclerosis (MS) associated with remote Epstein–Barr virus (EBV) infection. Data from [22–24]. For Pohl, 2006, odds ratio estimated by the authors based on data provided in the original paper.
In sharp contrast with the low MS risk in EBV-negative individuals, risk of MS is increased among adults with history of mononucleosis, which is the common clinical manifestation of EBV infection in adolescence and adulthood [21,25]. Compared with individuals infected with EBV in early childhood, MS risk is about 10-fold lower among EBV-negative individuals, and about two- to threefold higher among those infected with EBV later in life (as inferred from history of mononucleosis) – thus there is at least a 20-fold increase in risk among individuals with a history of mononucleosis compared to those who are EBV-negative, in spite of their sharing a similar high-hygiene childhood environment (Fig. 2).
Anti-EBV antibodies and risk of MS
Corroborating a role of EBV as an aetiological agent in MS is the now well-established observation that anti-EBV antibodies, but not of antibodies to other herpes viruses, becomes elevated significantly 5 or more years before the onset of symptoms. The most consistent finding is an elevation of antibodies against EBV nuclear antigens (EBNA complex and EBNA 1) expressed during latent infection, and only a marginal or absent elevation of antibodies to the viral capsid protein expressed during the lytic cycle (Fig. 4) [26–29].
Fig. 4.
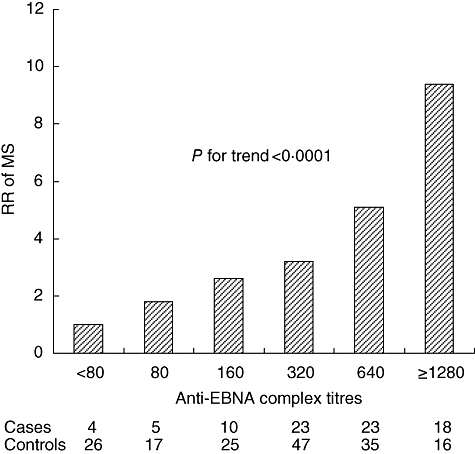
Relative risk of multiple sclerosis (MS) according to serum levels of anti-Epstein–Barr virus EBV nuclear antigens (EBNA) antibody titres an average of 4 years before the onset of symptoms. Data from [28].
The relation between anti-EBNA antibodies and MS risk is not explained by confounding by genetic factors – an increasing risk of MS with increasing anti-EBV titres is observed in human leucocyte antigen (HLA)-DRB1*15-negative as well as in HLA-DRB1*15-positive individuals. The DR15 allele and anti-EBNA titres seem to act as independent risk factors with multiplicative effects [30]. Further, in a longitudinal study, adjustment for anti-EBNA titres attenuated the association between anti-myelin antibodies and MS risk, suggesting that there may be some cross-reactivity between EBNA-1 and myelin peptides [31].
Beyond EBV – what remains unexplained
A role of EBV in MS causation could explain the remarkable similarity between the epidemiology of MS and mononucleosis, including age of peak incidence, latitude gradient, rarity in populations in which EBV infections occur early in life (including Japan and most of Asia), earlier age at peak onset in women than men, lower incidence in blacks, Asian and Eskimos than in whites and positive correlation with socio-economic status [7,32]. The early age at EBV infection could also explain the low MS risk among people who migrate from areas of low MS prevalence to areas of high prevalence. A robust observation in MS epidemiology that cannot be explained by EBV, however, is the marked reduction in risk associated with migration from high to low MS prevalence areas. This finding suggests that other factors may be at play. For example, the effect of EBV infection on MS risk may be modified by other environmental factors, such as the vitamin D status of the host, which depends upon sunlight exposure and thus correlates strongly with latitude [33,34], or by co-infection with a microbe that increases (and is more common in high MS risk areas) or decreases (and is more common in low-risk areas) MS risk. It is also possible that EBV itself varies across regions, and some EBV strains may be more likely to increase MS risk than others [35]. The possibility that there is an MS-related EBV strain has so far been little investigated, but some supportive evidence has been reported [36].
EBV and MS pathology
A major obstacle to the broad acceptance of a role of EBV in MS aetiology has been the putative absence of EBV itself from MS lesions [37,38]. Aloisi and colleagues have recently challenged this notion by reporting the presence of large numbers of EBV-infected B cells in the brain of a large majority of patients with MS [39]. These cells were more numerous in areas with active inflammatory infiltrates, where cytotoxic CD8+ T cells displaying an activated phenotype were seen contiguous to the EBV-infected cells. These findings, however, have not been confirmed in a recent investigation [40], and whether or not EBV is involved directly in the pathogenesis of MS lesions remains uncertain.
The strong epidemiological evidence linking EBV to MS is, however, independent from the pathological findings. If the presence of EBV in the central nervous system (CNS) of MS patients is not confirmed, mechanisms other than CNS infection would have to be identified [41,42]. These may include cross-reactivity between EBV and myelin antigens [43–45] and antibodies [43–46]. A molecular mimicry mechanism is supported by the discovery that MS patients have an increased frequency and broadened specificity of CD4+ T cells recognizing EBNA 1 [47], the identification of EBV peptides as targets of the immune response in the cerebrospinal fluid (CSF) of some MS patients [48] and the isolation of EBNA-1-specific cell lines from MS patients that cross-react with myelin antigens [49]. Other proposed mechanisms include the activation of superantigens [50,51], increased expression of alpha B-crystallin [52,53] or infection of autoreactive B lymphocytes [54].
Summary
Although EBV is ubiquitous, the age at EBV infection is very variable and correlated strongly with level of hygiene during childhood. If high level of hygiene was a risk factor for MS, young adults not infected with EBV would be expected to have a high MS risk. The consistent observation in epidemiological studies that their MS risk is extremely low, but increases at least 20-fold among individuals who develop mononucleosis, is thus a strong rebuttal of the general formulation of the hygiene hypothesis and supports the notion that high hygiene increases MS risk by delaying the age at infection with EBV, which is itself related causally to MS.
Acknowledgments
We thank Ms Leslie Unger for technical assistance. Dr Ascherio's work on the epidemiology of MS is funded by the National Institute of Neurological Disorders and Stroke, National Institutes of Health grants RO1 NS042194 and RO1 NS046635.
Disclosure
The authors have nothing to disclose.
References
- 1.Koch-Henriksen N, Hyllested K. Epidemiology of multiple sclerosis: incidence and prevalence rates in Denmark 1948–64 based on the Danish Multiple Sclerosis Registry. Acta Neurol Scand. 1988;78:369–80. doi: 10.1111/j.1600-0404.1988.tb03672.x. [DOI] [PubMed] [Google Scholar]
- 2.Hernán MA, Olek MJ, Ascherio A. Geographic variation of MS incidence in two prospective studies of US women. Neurology. 1999;53:1711–18. doi: 10.1212/wnl.53.8.1711. [DOI] [PubMed] [Google Scholar]
- 3.Kurtzke JF. MS epidemiology world wide. One view of current status. Acta Neurol Scand. 1995;161(Suppl.):23–33. doi: 10.1111/j.1600-0404.1995.tb05853.x. [DOI] [PubMed] [Google Scholar]
- 4.Pugliatti M, Sotgiu S, Rosati G. The worldwide prevalence of multiple sclerosis. Clin Neurol Neurosurg. 2002;104:182–91. doi: 10.1016/s0303-8467(02)00036-7. [DOI] [PubMed] [Google Scholar]
- 5.Gale CR, Martyn CN. Migrant studies in multiple sclerosis. Prog Neurobiol. 1995;47:425–48. [PubMed] [Google Scholar]
- 6.Kurtzke JF, Beebe GW, Norman JE. Epidemiology of multiple sclerosis in US veterans: III. Migration and the risk of MS. Neurology. 1985;35:672–8. doi: 10.1212/wnl.35.5.672. [DOI] [PubMed] [Google Scholar]
- 7.Ascherio A, Munger KL. Environmental risk factors for multiple sclerosis. Part I: the role of infection. Ann Neurol. 2007;61:288–99. doi: 10.1002/ana.21117. [DOI] [PubMed] [Google Scholar]
- 8.Poskanzer DC, Schapira K, Miller H. Multiple sclerosis and poliomyelitis. Lancet. 1963;2:917–21. doi: 10.1016/s0140-6736(63)90624-x. [DOI] [PubMed] [Google Scholar]
- 9.Okada H, Kuhn CH, Feillet H, Bach J-F. The ‘hygiene hypothesis’ for autoimmune and allergic diseases: an update. Clin Exp Immunol. 2010;160:1–9. doi: 10.1111/j.1365-2249.2010.04139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leibowitz U, Antonovsky A, Medalie JM, Smith HA, Halpern L, Alter M. Epidemiological study of multiple sclerosis in Israel. II. Multiple sclerosis and level of sanitation. J Neurol Neurosurg Psychiatry. 1966;29:60–8. doi: 10.1136/jnnp.29.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med. 2002;347:911–20. doi: 10.1056/NEJMra020100. [DOI] [PubMed] [Google Scholar]
- 12.Hafler DA. The distinction blurs between an autoimmune versus microbial hypothesis in multiple sclerosis [Comment] J Clin Invest. 1999;104:527–9. doi: 10.1172/JCI8193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hunter SF, Hafler DA. Ubiquitous pathogens: links between infection and autoimmunity in MS? [Editorial; Comment] Neurology. 2000;55:164–5. doi: 10.1212/wnl.55.2.164. [DOI] [PubMed] [Google Scholar]
- 14.Beebe GW, Kurtzke JF, Kurland LT, Auth TL, Nagler B. Studies on the natural history of multiple sclerosis. 3. Epidemiologic analysis of the Army experience in World War II. Neurology. 1967;17:1–17. doi: 10.1212/wnl.17.1.1. [DOI] [PubMed] [Google Scholar]
- 15.Kurtzke JF, Page WF. Epidemiology of multiple sclerosis in US veterans: VII. Risk factors for MS. Neurology. 1997;48:204–13. doi: 10.1212/wnl.48.1.204. [DOI] [PubMed] [Google Scholar]
- 16.Russell WR. Multiple sclerosis: occupation and social group at onset. Lancet. 1971;2:832–4. doi: 10.1016/s0140-6736(71)90216-9. [DOI] [PubMed] [Google Scholar]
- 17.Granieri E, Casetta I. Part III: selected reviews. Common childhood and adolescent infections and multiple sclerosis. Neurology. 1997;49(Suppl. 2):S42–S54. doi: 10.1212/wnl.49.2_suppl_2.s42. [DOI] [PubMed] [Google Scholar]
- 18.Ponsonby AL, van der Mei I, Dwyer T, et al. Exposure to infant siblings during early life and risk of multiple sclerosis. JAMA. 2005;293:463–9. doi: 10.1001/jama.293.4.463. [DOI] [PubMed] [Google Scholar]
- 19.Wallin MT, Page WF, Kurtzke JF. Multiple sclerosis in US veterans of the Vietnam era and later military service: race, sex, and geography. Ann Neurol. 2004;55:65–71. doi: 10.1002/ana.10788. [DOI] [PubMed] [Google Scholar]
- 20.Henle W, Henle G. 4. Seroepidemiology of the virus. In: Epstein MA, Achong BG, editors. The Epstein–Barr virus. Berlin: Springer; 1979. pp. 61–78. [Google Scholar]
- 21.Thacker EL, Mirzaei F, Ascherio A. Infectious mononucleosis and risk for multiple sclerosis: a meta-analysis. Ann Neurol. 2006;59:499–503. doi: 10.1002/ana.20820. [DOI] [PubMed] [Google Scholar]
- 22.Alotaibi S, Kennedy J, Tellier R, Stephens D, Banwell B. Epstein–Barr virus in pediatric multiple sclerosis. JAMA. 2004;291:1875–9. doi: 10.1001/jama.291.15.1875. [DOI] [PubMed] [Google Scholar]
- 23.Pohl D, Krone B, Rostasy K, et al. High seroprevalence of Epstein–Barr virus in children with multiple sclerosis. Neurology. 2006;67:2063–5. doi: 10.1212/01.wnl.0000247665.94088.8d. [DOI] [PubMed] [Google Scholar]
- 24.Banwell B, Krupp L, Kennedy J, et al. Clinical features and viral serologies in children with multiple sclerosis: a multinational observational study. Lancet Neurol. 2007;6:773–81. doi: 10.1016/S1474-4422(07)70196-5. [DOI] [PubMed] [Google Scholar]
- 25.Nielsen TR, Rostgaard K, Nielsen NM, et al. Multiple sclerosis after infectious mononucleosis. Arch Neurol. 2007;64:72–5. doi: 10.1001/archneur.64.1.72. [DOI] [PubMed] [Google Scholar]
- 26.Ascherio A, Munger KL, Lennette ET, et al. Epstein–Barr virus antibodies and risk of multiple sclerosis: a prospective study. JAMA. 2001;286:3083–8. doi: 10.1001/jama.286.24.3083. [DOI] [PubMed] [Google Scholar]
- 27.Sundstrom P, Juto P, Wadell G, et al. An altered immune response to Epstein–Barr virus in multiple sclerosis: a prospective study. Neurology. 2004;62:2277–82. doi: 10.1212/01.wnl.0000130496.51156.d7. [DOI] [PubMed] [Google Scholar]
- 28.Levin LI, Munger KL, Rubertone MV, et al. Temporal relationship between elevation of Epstein–Barr virus antibody titers and initial onset of neurological symptoms in multiple sclerosis. JAMA. 2005;293:2496–500. doi: 10.1001/jama.293.20.2496. [DOI] [PubMed] [Google Scholar]
- 29.DeLorenze GN, Munger KL, Lennette E, Orentreich N, Vogelman J, Ascherio A. Epstein–Barr virus and multiple sclerosis: evidence of association from a prospective study with long-term follow-up. Arch Neurol. 2006;63:839–44. doi: 10.1001/archneur.63.6.noc50328. [DOI] [PubMed] [Google Scholar]
- 30.De Jager PL, Simon KC, Munger KL, Rioux JD, Hafler DA, Ascherio A. Integrating risk factors: HLA-DRB1*1501 and Epstein–Barr virus in multiple sclerosis. Neurology. 2008;70:1113–18. doi: 10.1212/01.wnl.0000294325.63006.f8. [DOI] [PubMed] [Google Scholar]
- 31.Wang H, Munger KL, Reindl M, et al. Myelin oligodendrocyte glycoprotein antibodies and multiple sclerosis in healthy young adults. Neurology. 2008;71:1142–6. doi: 10.1212/01.wnl.0000316195.52001.e1. [DOI] [PubMed] [Google Scholar]
- 32.Warner HB, Carp RI. Multiple sclerosis and Epstein–Barr virus (Letter) Lancet. 1981;2:1290. doi: 10.1016/s0140-6736(81)91527-0. [DOI] [PubMed] [Google Scholar]
- 33.Ascherio A, Munger KL. Environmental risk factors for multiple sclerosis. Part II: noninfectious factors. Ann Neurol. 2007;61:504–13. doi: 10.1002/ana.21141. [DOI] [PubMed] [Google Scholar]
- 34.Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004;80(Suppl.):1678S–88S. doi: 10.1093/ajcn/80.6.1678S. [DOI] [PubMed] [Google Scholar]
- 35.Gratama JW, Ernberg I. Molecular epidemiology of Epstein–Barr virus infection. Adv Cancer Res. 1995;67:197–255. [PubMed] [Google Scholar]
- 36.Munch M, Hvas J, Christensen T, Møller-Larsen A, Haahr S. A single subtype of Epstein–Barr virus in members of multiple sclerosis clusters. Acta Neurol Scand. 1998;98:395–9. doi: 10.1111/j.1600-0404.1998.tb07320.x. [DOI] [PubMed] [Google Scholar]
- 37.Hilton DA, Love S, Fletcher A, Pringle JH. Absence of Epstein–Barr virus RNA in multiple sclerosis as assessed by in situ hybridisation. J Neurol Neurosurg Psychiatry. 1994;57:975–6. doi: 10.1136/jnnp.57.8.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanders VJ, Felisan S, Waddell A, Tourtellotte WW. Detection of herpesviridae in postmortem multiple sclerosis brain tissue and controls by polymerase chain reaction. Neurovirology. 1996;2:249–58. doi: 10.3109/13550289609146888. [DOI] [PubMed] [Google Scholar]
- 39.Serafini B, Rosicarelli B, Franciotta D, et al. Dysregulated Epstein–Barr virus infection in the multiple sclerosis brain. J Exp Med. 2007;204:2899–912. doi: 10.1084/jem.20071030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Willis SN, Stadelmann C, Rodig SJ, et al. Epstein–Barr virus infection is not a characteristic feature of multiple sclerosis brain. Brain. 2009;132:3318–28. doi: 10.1093/brain/awp200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cook SD. Does Epstein–Barr virus cause multiple sclerosis? Rev Neurol Dis. 2004;1:115–23. [PubMed] [Google Scholar]
- 42.Ercolini AM, Miller SD. The role of infections in autoimmune disease. Clin Exp Immunol. 2009;155:1–15. doi: 10.1111/j.1365-2249.2008.03834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lang HL, Jacobsen H, Ikemizu S, et al. A functional and structural basis for TCR cross-reactivity in multiple sclerosis. Nat Immunol. 2002;3:940–3. doi: 10.1038/ni835. [DOI] [PubMed] [Google Scholar]
- 44.Hollsberg P, Hansen HJ, Haahr S. Altered CD8+T cell responses to selected Epstein–Barr virus immunodominant epitopes in patients with multiple sclerosis. Clin Exp Immunol. 2003;132:137–43. doi: 10.1046/j.1365-2249.2003.02114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holmoy T, Vartdal F. Cerebrospinal fluid T cells from multiple sclerosis patients recognize autologous Epstein–Barr virus-transformed B cells. J Neurovirol. 2004;10:52–6. doi: 10.1080/13550280490261671. [DOI] [PubMed] [Google Scholar]
- 46.Rand KH, Houck H, Denslow ND, Heilman KM. Epstein–Barr virus nuclear antigen-1 (EBNA-1) associated oligoclonal bands in patients with multiple sclerosis. J Neurol Sci. 2000;173:32–9. doi: 10.1016/s0022-510x(99)00298-1. [DOI] [PubMed] [Google Scholar]
- 47.Lunemann JD, Edwards N, Muraro PA, et al. Increased frequency and broadened specificity of latent EBV nuclear antigen-1-specific T cells in multiple sclerosis. Brain. 2006;129:1493–506. doi: 10.1093/brain/awl067. [DOI] [PubMed] [Google Scholar]
- 48.Cepok S, Zhou D, Srivastava R, et al. Identification of Epstein–Barr virus proteins as putative targets of the immune response in multiple sclerosis. J Clin Invest. 2005;115:1352–60. doi: 10.1172/JCI23661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lunemann JD, Jelcic I, Roberts S, et al. EBNA1-specific T cells from patients with multiple sclerosis cross react with myelin antigens and co-produce IFN-{gamma} and IL-2. J Exp Med. 2008;205:1763–73. doi: 10.1084/jem.20072397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sutkowski N, Palkama T, Ciurli C, Sekaly R-P, Thorley-Lawson DA, Huber BT. An Epstein–Barr virus-associated superantigen. J Exp Med. 1996;184:971–80. doi: 10.1084/jem.184.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tai A, O'Reilly E, Alroy K, et al. Human endogenous retrovirus-K18 Env as a risk factor in multiple sclerosis. Mult Scler. 2008;14:1175–80. doi: 10.1177/1352458508094641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Sechel AC, Bajramovic JJ, van Stipdonk MJ, Persoon-Deen C, Geutskens SB, van Noort JM. EBV-induced expression and HLA-DR-restricted presentation by human B cells of alpha B-crystallin, a candidate autoantigen in multiple sclerosis. J Immunol. 1999;162:129–35. [PubMed] [Google Scholar]
- 53.Ousman SS, Tomooka BH, van Noort JM, et al. Protective and therapeutic role for alphaB-crystallin in autoimmune demyelination. Nature. 2007;448:474–9. doi: 10.1038/nature05935. [DOI] [PubMed] [Google Scholar]
- 54.Pender MP. Infection of autoreactive B lymphocytes with EBV, causing chronic autoimmune diseases. Trends Immunol. 2003;24:584–8. doi: 10.1016/j.it.2003.09.005. [DOI] [PubMed] [Google Scholar]


