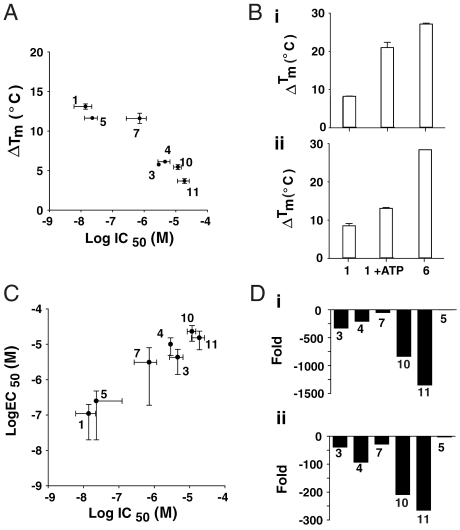
Fig. 4.
Correlation between stabilization and inhibitor potency. Compounds are identified in Table 1. (A) The stabilizing effect of PTC124 analogs, as demonstrated by ΔTm, on 3.6 μM Fluc in the presence of 2 mM ATP correlates with their IC50s for FLuc inhibition in vitro. The average maximum ΔTm is plotted and error bars represent the SD from two determinations. Compounds showing decreasing ΔTm also show lower inhibitor potency against the enzyme. (B) The ΔTm values for PTC124 (1), PTC 124 + 2 mM ATP, and the synthetically prepared PTC124-AMP adduct (6) are shown in either PBS (i) or Tris-acetate buffer (ii). The higher ΔTm value obtained for the synthetic adduct is likely due to the fact that this adduct is measured in the absence of ATP (2 mM). Adding an equivalent concentration of ATP to 6 yielded the same ΔTm value (12.6 °C) as was observed with PTC124 and ATP. (C) A correlation plot of the EC50 values in the cell-based FLuc nonsense codon suppression assay plotted against their IC50 values in the purified FLuc enzymatic assay for PTC124, a regioisomer, and related analogs (values from Table 1). Inhibition of purified FLuc in an enzymatic assay follows the same trend as apparent activation in a cell-based FLuc reporter gene assay. (D) Fold-shift in IC50 values for PTC124 analogs relative to PTC124 (1) in the FLuc enzyme assay (i) and the nonsense codon suppression cell-based assay (ii) indicate that the potency trends remain largely similar in both types of assay.