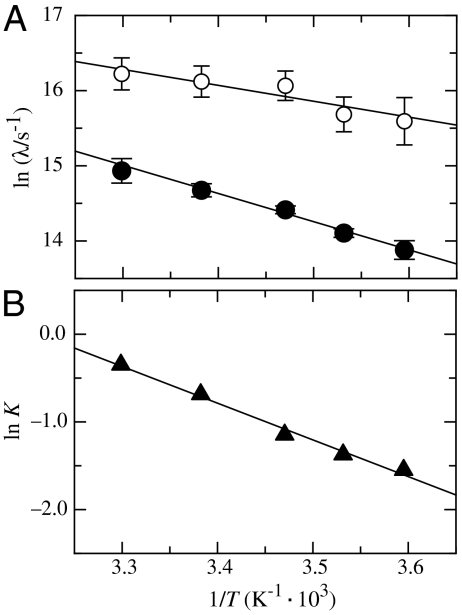
Fig. 3.
(A) Teperature dependence of the fast (open circles) and the slow (solid circles) native-state TTET process in the Nal23/Xan35 variant at zero denaturant. A fit to the Arrhenius equation (see SI Text) gives A = 1.3·1010 s-1 and Ea = 17 ± 1 kJ/mol for the faster reaction and A = 7.5·1011 s-1 and Ea = 32 ± 1 kJ/mol are the slower process. (B) Van’t Hoff plot for the equilibrium between N and N′. The equilibrium constant (K = [N′]/[N]) was determined by the ratio of the amplitudes of the fast and the slow native-state TTET process. A fit to the van’t Hoff equation (see SI Text) gives ΔH0 = 35 ± 4 kJ/mol and ΔS0 = 112 ± 20 J·mol-1·K-1.