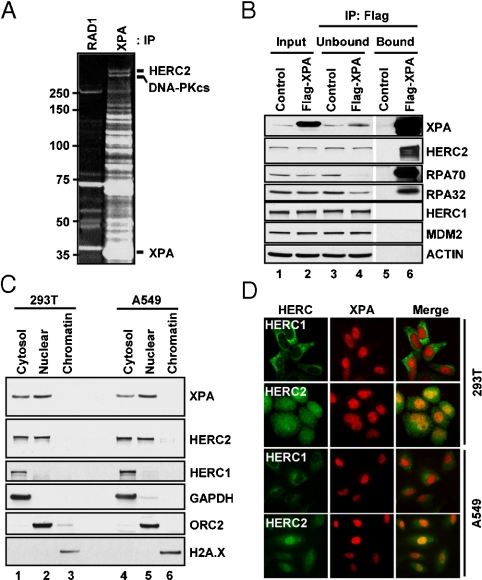
Fig. 4.
Interaction of XPA with the putative ubiquitin E3 ligase HERC2. (A) HEK293 cells stably expressing either Flag-tagged XPA or Flag-tagged RAD1 (control) were induced to express the corresponding proteins. The cell lysates were then mixed with anti-Flag agarose beads and the bound proteins were visualized by SDS-PAGE and SYPRO Ruby staining. The XPA-interacting proteins were identified by mass spectroscopy. For clarity, we indicate only the bands corresponding to XPA, HERC2, and DNA-PKcs (which migrates like a doublet of HERC2). (B) XPA was immunoprecipitated from extract of either Flag-XPA or mock (control) expressing cells with anti-Flag beads and the unbound and bound fractions were probed with the appropriate antibodies. The input lanes contain 5% of the input protein. (C) Two cell lines were fractionated into “cytosol,” “nuclear,” and “chromatin” fractions and the distribution of XPA, HERC2, HERC1, and markers for the three subcellular fractions GAPDH (cytosolic), ORC2 (nuclear), H2A.X (chromatin) were analyzed by immunoblotting. (D) 293T and A549 cells were permeablized, fixed, stained with XPA and either HERC1 or HERC2 antibodies (Fig. S6), and imaged using a fluorescent microscope.