Abstract
The role of intraspecific and interspecific interactions in structuring biotic communities at fine spatial scales is well documented, but the signature of species interactions at coarser spatial scales is unclear. We present evidence that species interactions may be a significant factor in mediating the regional assembly of the Danish avifauna. Because >95% of breeding species (n = 197) are migratory, we hypothesized that dispersal limitation would not be important and that breeding distributions would largely reflect resource availability and autecological habitat preferences. Instead, we detected a striking pattern of spatial segregation between ecologically similar species at two spatial scales with a suite of null models that factored in the spatial distribution of habitats in Denmark as well as population size and biomass of each species. Habitat utilization analyses indicated that community-wide patterns of spatial segregation could not be attributed to the patchy distribution of habitat or to gross differences in habitat utilization among ecologically similar species. We hypothesize that, when habitat patch size is limited, conspecific attraction in concert with interspecific territoriality may result in spatially segregated distributions of ecologically similar species at larger spatial scales. In the Danish avifauna, the effects of species interactions on community assembly appear pervasive and can be discerned at grain sizes up to four orders of magnitude larger than those of individual territories. These results suggest that species interactions should be incorporated into species distribution modeling algorithms designed to predict species occupancy patterns based on environmental variables.
Keywords: null models, assembly rules, interspecific territoriality, conspecific social attraction, allee affect
The study of species interactions has been at the forefront of ecological research for 75 years (1–4), but the range of spatial scales at which interactions may be discerned in natural communities is imperfectly known. Species interactions affect the fine-grained spacing of individuals in a wide range of organisms including plants (5, 6), marine invertebrates (7–9), social insects (10), fish (11), lizards (12), and mammals (13). The evidence is particularly good for birds, where aggressive interactions may result in interspecific territoriality in which individuals defend territories against both conspecific and heterospecific individuals (14–16). At what point along the spatial continuum from individual territories to continental landscapes does the signature of species interactions cease to be visible?
Interspecific competition can have a pervasive influence on the distribution, abundance, and foraging behavior of birds on small islands (17–19), and it has been hypothesized that local competition among species could “scale up” to generate competitively driven distributional patterns on larger islands (20). However, interspecific competition has a more subtle and ecologically limited effect in mainland avifaunas (14–16, 21). The extent to which interspecific competition influences the geographic distribution of species in continental landscapes has never been resolved. Because large-scale field experiments on avian communities are unfeasible, evidence of interspecific competition has been sought in binary presence/absence matrices of species occurrences on islands (20, 22) and in continental mainland regions (23). Inferences of community assembly rules from statistical analyses of presence/absence data are controversial. Even with the use of sophisticated null-model analyses, it is not possible in most systems to discriminate spatial patterns generated by species interactions from those caused by historical effects, dispersal barriers, and especially those resulting from habitat selection, the intrinsic preferences that species show for particular habitats (24). Large-scale distributional signals of species interactions, if they exist in continental avifaunas, originate at the scale of individual territories. Although habitat selection manifests itself at a wide range of grain sizes (24, 25), the effects of intraspecific and interspecific interactions in continental landscapes previously have been detected only at small grain sizes (14–16, 21, 24, 26–29). In this paper, we present evidence that both intraspecific and interspecific interactions may influence the large-scale spatial distribution of breeding birds in Denmark.
Denmark consists of the Jylland Peninsula and an archipelago of land-bridge islands, most of which are visible from the mainland. The contemporary breeding avifauna (197 species) is largely migratory, and only a handful of species (<5%) can be classified as sedentary residents, although juveniles of even these species disperse widely (30). A majority of migratory species also have breeding populations in Sweden and Norway that transit Denmark during migration. Thus, the breeding distribution of birds in Denmark largely reflects resource availability, habitat selection, and the outcome of species interactions, rather than dispersal limitation, historical contingency, or evolutionary processes (none of the species in this assemblage are endemic to Denmark).
To disentangle the effects of species interactions from those of habitat selection in the Danish avifauna, we analyzed the breeding distributions of birds at two spatial grains—from a gridded matrix of 5-km × 5-km cells (n = 2003) and a larger-scale aggregation of 10-km × 10-km cells (n = 620) (Fig. 1 and Fig. S1). Cells of the smaller grain size (25 km2) are roughly equivalent in area to the breeding territories of the largest raptors (e.g., Bubo bubo) but are three to four orders of magnitude larger than the breeding territories of songbirds, which numerically dominate the Danish avifauna. We then quantified the areas of principal terrestrial and aquatic habitats occurring in each cell at the two spatial scales (Table S1). These complementary databases were used to analyze the co-occurrence patterns of species and the observed and expected values of habitat utilization and electivity at two nested levels of assemblage organization: (i) foraging guilds within the avifauna and (ii) sets of congeneric or closely related species within foraging guilds. This hierarchical framework groups species into guilds of ecologically similar species, with congeneric species within foraging guilds exhibiting the greatest similarity in foraging behavior and morphology.
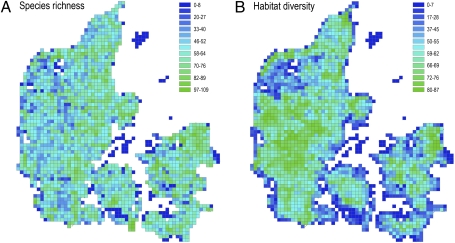
Fig. 1.
Species richness of Danish breeding birds (Left) and spatial variation in habitat diversity (HD) (Right) of grid cells at a grain size of 5 km × 5 km (25 km2). The HD score is the product of relative grid cell area and the probability that two points randomly chosen within a grid cell represent different habitat types (54). The HD score was used to parameterize null models of random species colonization independently. Species richness ranged from 1 to 109 species per cell (16, 60). The best-fitting power function was S = 27.93681(HD)0.1916, r2 = 0.1171. See Fig. S1 for comparable figures at the 10-km × 10-km (100-km2) grain size.
We crossed this spatial and guild classification with analyses of four null models of species co-occurrence: a standard “fixed-fixed” null model (which preserves row and column sums of the observed binary presence/absence matrix) and three additional models that used information on habitat availability, population sizes, and biomass to modify marginal probability distributions (Table S1, S2, and S3 and Figs. S2 and S3). Finally, we conducted null-model analyses of habitat utilization and electivity (31, 32) at both grain sizes for the foraging and congeneric guilds. The resulting suite of 24 sets of null-model analyses (two guild categories × two grain sizes × six null models) permits us to address two fundamental questions about the distributional patterns of Danish breeding birds: (i) Do species in foraging and congeneric guilds exhibit nonrandom patterns of spatial aggregation or segregation? (ii) Can nonrandom distributional patterns at different spatial scales be accounted for by the availability and selection of habitat?
Results
Co-Occurrence Patterns Within Foraging Guilds.
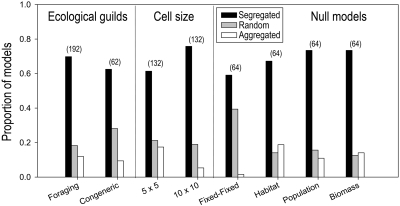
Species within most foraging guilds exhibited segregated distributions (Fig. 2, Left and Table S4). Summed across all of the foraging guilds, null models, and spatial grain sizes (24 guilds × 2 grain sizes × 4 null models = 192 analyses), 69.8% of tests indicated statistically significant segregated distributions, 18.2% showed random distributions, and 12.0% indicated statistically significant aggregated distributions. In a comparison of patterns at the two grain sizes, a greater fraction of tests indicated segregated distributions in 100-km2 cells (74 segregated, 6 aggregated) than in 25-km2 cells (60 segregated, 18 aggregated). In a comparison of the different null models, combining results from both scales of resolution, all four indicated relatively high frequencies of segregated patterns: fixed-fixed model (29 segregated, 0 aggregated); habitat model (33 segregated, 9 aggregated), population model (36 segregated, 6 aggregated); and biomass model (36 segregated, 7 aggregated). The habitat model showed the greatest difference in patterns between 100-km2 cells (22 segregated, 1 aggregated) and 25-km2 cells (11 segregated, 8 aggregated).
Fig. 2.
Summary of null-model analyses of species co-occurrence in ecological guilds of Danish birds (Tables S4 and S5).
Four foraging guilds exhibited segregated distributional patterns at both spatial grains over all models, whereas 11 guilds exhibited a mixture of segregated and random distributions (Table S4). Eight guilds exhibited a mixture of segregated, aggregated, and random distributions, but only the dabbling ducks showed a strong pattern of aggregation (three of four models at both grain sizes). Of particular interest, the eight foraging guilds composed almost entirely of territorial songbirds (openland insectivores, terrestrial and low-stratum flycatchers, thrushes, marsh warblers, foliage gleaners, tit-like birds, corvids, passerine seedeaters) showed strongly segregated distributions in 25-km2 cells (20 segregated, 6 random, 5 aggregated) and 100-km2 cells (24 segregated, 7 random, 1 aggregated).
Co-Occurrence Patterns Within Congeneric Guilds.
Segregated patterns of distributional overlap in congeneric guilds of territorial songbirds provided further confirmation of patterns observed in foraging guilds (Fig. 2, Left and Table S5). Summed across congeneric guilds and both spatial grains (eight guilds × four null models × two grain sizes = 64 analyses), 62.5% of tests indicated statistically significant segregated distributions, 28.1% showed random distributions, and 9.4% indicated statistically significant aggregated distributions. A greater fraction of tests indicated segregated distributions in 100-km2 cells (21 segregated, 1 aggregated) than in 25-km2 cells (18 segregated, 5 aggregated). All null models indicated relatively high frequencies of segregated patterns: 8 segregated and 1 aggregated for the fixed-fixed model; 10 segregated and 2 aggregated for the habitat model; 11 segregated and 1 aggregated for the population model; and 10 segregated and 2 aggregated for the biomass model.
Summing across spatial grain sizes, four congeneric guilds exhibited a mixture of segregated and random distributions; the remaining four guilds showed a mixture of segregated, random, and aggregated distributions (Table S5). Overlap patterns in Sylvia (2 segregated, 4 random, 2 aggregated) and Phylloscopus (1 segregated, 5 random, 2 aggregated) were equivocal. The remaining six guilds showed strong patterns of spatial segregation: Anthus (6 segregated, 1 random, 1 aggregated); Acrocephalus (6 segregated, 2 random, 0 aggregated); Parus (5 segregated, 2 random, 1 aggregated); Corvus (8 segregated, 0 random, 0 aggregated); Carduelis (6 segregated, 2 random, 0 aggregated); and Turdus (6 segregated, 2 random, 0 aggregated).
Habitat Utilization and Electivity Within Foraging Guilds.
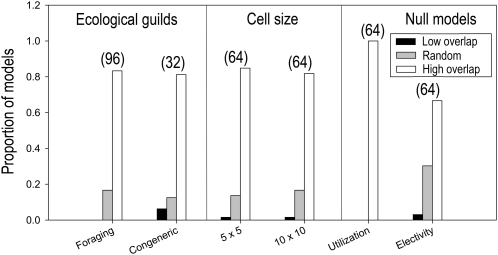
All foraging guilds showed significantly high overlap in habitat utilization at both spatial grain sizes (48/48 tests; Table S6). Similar patterns of high overlap were observed in habitat electivity analyses of 25-km2 cells (17/24 tests) and 100-km2 cells (15/24 tests). Species within foraging guilds never exhibited mutually exclusive patterns of habitat utilization and electivity. These analyses suggest that the pervasive spatial patterns of segregation indicated by the four co-occurrence null models (Fig. 2) were not caused by checkerboard distributions of habitats or by gross differences among species in habitat preferences.
Habitat Utilization and Electivity Within Congeneric Guilds.
Congeneric guilds are composed of species that might be expected, a priori, to exhibit the greatest degree of niche overlap based on phylogenetic similarity and niche conservatism. Congeneric guilds showed significantly high overlap in habitat utilization at both spatial grain sizes (16/16 tests; Table S7). Similar patterns of high overlap were observed in habitat electivity in 25-km2 cells (six of eight tests) and in 100-km2 cells (six of eight tests). The one exception was observed in Sylvia (five species), which exhibited high overlap in habitat utilization but mutually exclusive patterns of habitat electivity at both spatial grains. This result suggests that species of sylviid warblers occupy cells with a similar spectrum of common habitats but may differ from one another in their occupancy of grid cells containing uncommon habitats (i.e., shrublands and deciduous woodlands).
Discussion
We began the analyses with the expectation that the breeding distribution of birds in Denmark would be linked in a simple way to the availability of preferred habitat at the scale of analysis (Fig. 1) (33). The significant aggregation of dabbling ducks in grid cells containing marsh and freshwater lakes, for example, was consistent with this expectation. We were surprised, however, to discover a pervasive pattern of spatial segregation of species belonging to well-defined foraging and congeneric guilds (Fig. 2), especially among species of territorial songbirds. Because terrestrial habitat diversity is high within 25-km2 grid cells (9.6 of a possible 10 habitats), there is little evidence that segregated patterns of spatial overlap among widely distributed territorial species are caused by checkerboard distributions of distinctive habitat types or reflect strong differences among species in habitat preferences (Fig. 3). A lack of habitat sorting also was confirmed by the pattern of high overlap in habitat utilization and electivity among species belonging to the same foraging and congeneric guilds (Tables S6 and S7). The one exception was observed in Sylvia warblers, which exhibited significantly less overlap in habitat electivity. Although these findings do not rule out the possibility that subtle habitat preferences influence the pattern of spatial segregation among other guilds at coarser spatial scales, they do suggest that behavioral factors other than simple habitat selection may influence the spatial distributions of species at grain sizes several orders of magnitude larger than the areas of individual territories.
Fig. 3.
Summary of null-model analyses of niche overlap in habitat utilization and electivity in ecological guilds of Danish birds (Tables S6 and S7).
Conspecific and heterospecific attraction often result in clumped or aggregated distributions of breeding birds, most notably among colonial species such as herons, gulls, and swallows (34, 35). The occurrence of conspecific and heterospecific attraction among songbirds that defend relatively large territories (0.1–10 ha) is arguably more intriguing because the adaptive advantages of aggregated distributions for highly territorial species are less apparent. Because heterospecific attraction would yield a significant excess of aggregated distributions among pairs of species (36), the opposite of what we observed, it may be excluded as the basis for the pervasive community-wide patterns of spatial segregation.
Although logistical and ethical constraints prevented us from conducting large-scale field experiments, we hypothesize that the underlying cause of spatial segregation in territorial species at larger scales of resolution stems primarily from conspecific attraction. Several field studies have shown that patch suitability is enhanced by the presence of conspecifics, which can lead to local abundance peaks higher than expected from the distribution of habitat resources (34). The benefits of local aggregative behavior in territorial birds, including mate acquisition and public information sharing, are examples of Allee effects (37, 38), broadly defined as the positive relationship between fitness and the number of conspecifics. Allee effects, which often are manifest at low population densities, may result in conspecific aggregations at spatial scales larger than those of individual territories.
Although conspecific attraction may explain local aggregations of species at the grain sizes analyzed in this study, it cannot explain the excess frequency of interspecific segregation observed in many foraging and congeneric guilds of Danish birds. Interspecific territoriality has been documented in a number of territorial songbirds in Eurasia (21, 39–41), even among some pairs of distantly related species (42). However, spatially segregated territories occur most frequently within pairs of closely related, ecologically similar species that occupy structurally simple habitats (15, 21, 28). When interspecific territoriality occurs in heterogeneous or structurally diverse environments, behaviorally dominant species usually exclude less aggressive species from the more productive end of successional gradients, leading to local habitat segregation (14). It should be noted that similar patterns of habitat segregation commonly arise in the absence of competition through the mechanism of habitat selection in heterogeneous environments (24, 43–45). Although habitat patch size may be another determining factor for species occupancy, the minimum patch size for most northern European songbirds is relatively small (<1 ha) (46).
In the Danish avifauna, migratory species arrive to nearly empty habitat each spring. Annual mortality rates of migratory songbirds are relatively high (30), and a substantial fraction of arriving individuals are naïve yearlings with no prior breeding experience. Priority effects may come into play if several males of one species establish contiguous territories in a habitat patch before males of other species arrive. Conspecific attraction then might permit one species to dominate numerically a habitat patch so that it becomes less attractive to arriving heterospecifics, which either fail to establish territories or rapidly emigrate to other patches of similar habitat that support larger numbers of their own species. It thus is plausible that conspecific attraction combined with interspecific territoriality could result in mutually exclusive distributions of species at relatively large spatial scales. Interspecific territoriality alone would be unlikely to result in spatial segregation at the grain sizes studied here. The mechanism described above would be more likely to occur among migratory than resident species, at low rather than high population densities, and in patchy environments where patch size is relatively small. In summary, our analyses suggest that conspecific and heterospecific interactions can “scale up” to produce behaviorally driven assembly patterns at relatively large spatial grains. The next generation of coarse-grained macroecological studies may need to incorporate species interactions that occur at small spatial scales. Our results also suggest that a failure to incorporate mechanisms of species interactions may account for the mixed results of current species distribution modeling efforts that use only environmental variables to predict species occupancy (47, 48).
Methods
Geography.
The deglaciation of Denmark was completed 16,000–15,500 years ago (ybp) (49), and transformation of the region into the Jylland Peninsula (i.e., mainland) and an archipelago of nearby land-bridge islands took place ≈ 8,500 ybp through the rising of the Litorina Sea (50). Present-day Denmark (∼43,100 km2) presents an ideal geographic template for co-occurrence analysis of avian species at the regional scale. There are no major geographic barriers to avian dispersal (the highest point in Denmark is 173 m above sea level), and there is no evidence of in situ speciation (there are no endemic avian species or subspecies). The larger islands of Sjælland (7,016 km2), Fyn (2,977 km2), Lolland (1,241 km2), Falster (514 km2), Mors (363 km2), Als (314 km2), Langeland (284 km2), Møn (217 km2), Rømø (129 km2), Samsø (114 km2), Amager (90 km2), Ærø (88 km2), Tåsinge (70 km2), and Fanø (56 km2) were retained in our analyses. Islands with land and freshwater areas totaling <25 km2 and those occurring >20 km from Jylland or the principal land-bridge islands were omitted from the analyses.
Distributional Data.
The breeding distribution of the Danish avifauna was mapped at the resolution of 5-km × 5-km cells (25 km2), following the Universal Transverse Mercator coordinate system, by 750 observers during the period 1993–1996 (51) (see SI Text for additional sampling details). After small and distant islands and cells with <25 ha of land area were excluded from the data set, a total of 2,003 cells were available for analysis. We aggregated 5-km × 5-km cells (both complete and marginal) to create 10-km × 10-km cells (100 km2). At each grain size, we converted the distributional breeding records to a binary presence/absence (0,1) matrix in which rows represent species and columns represent cells. The matrix of 25-km2 cells supported 197 breeding species. Three species recorded during the 1993–1996 censuses (Ciconia ciconia, Tetrao tetrix, and Sylvia nisoria) no longer breed in Denmark. Two colonial species (Rissa tridactyla and Alca torda) that occurred in marginal coastal cells at the 25-km2 grain size were omitted from the matrix when scaling it up to 100-km2 cells (n = 620). Edge effects of peripheral cells were incorporated by taking account of the area of each cell and its habitat diversity, both of which are reduced in peripheral cells.
Habitat.
The Danish environment has experienced several millennia of intensive human disturbance (52) culminating in a contemporary terrestrial landscape characterized by fine-grained patchworks of heath, hedgerow, shrubland, and woodland embedded in a matrix of pasture, meadow, and cropland. Habitats within 25-km2 cells were previously classified into 12 distinctive categories defined and quantified based on remote sensing of 25-m × 25-m pixels (53): open saline water, open fresh water, urban and unvegetated ground, seasonally tilled cropland, grazed or mown grassland, marshland and bog, grassy heathland, mixed grassy and shrubby heathland, shrubby heathland, shrubby woodland, deciduous woodland, and coniferous woodland (Table S1). Cells typically contained a majority of the habitat categories present in Denmark (10.6 ± 1.0 of 12 possible habitats). We constructed a quantitative index of habitat heterogeneity (Fig. 1) based on the percent area of the common habitat categories occurring within 25-km2 cells. Habitat types covering <1% (25 ha) of the cell area were omitted from the diversity index for that cell. We estimated habitat heterogeneity (HH) as:
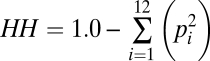 |
where pi is the proportion of the total area measured within each cell that is occupied by habitat i. This index measures the probability that two random points chosen within a cell represent two different habitats (54). HH can range from a minimum of 0.0 (if only a single habitat type is present) to a maximum of 0.917 (if all 12 habitats are equally common). At the 100-km2 grain size, we recalibrated the HH values of 13 cells (<3% of the total) from 0.00 to 0.01 so that relative probability weights could be calculated. We then multiplied HH by the cell area minus the area of open saline water to create an index of habitat diversity (HD). To minimize numerical round-off error in the HD index (which ranged from 0.01 to 83.77), 60 values <1.0 were rescaled to 1.0. Recalibration was unnecessary at the 25-km2 grain size.
Indices of Species-Specific Colonization Potential.
The ability of a species to colonize isolated patches of habitat is influenced by many factors including population size and dispersal behavior (53, 55). We did not attempt to model dispersal behavior per se, because the spatial scales of annual migration and natal dispersal distances of European birds are large relative to the grain size of census cells (30). Parasitism, disease, and predation also may influence the occupancy of habitat patches, but comprehensive data on these potentially important factors were unavailable.
We constructed two indices of colonization potential, one based on the estimated size of breeding populations in Denmark (51) and a second based on the biomass of each species (body mass × Danish population size). We estimated body mass as the midpoint of the mean values recorded for males and females, respectively (Table S2). Interspecific variation in avian body mass correlates with longevity (56), which in turn may be linked with a species’ ability to resist local extinction through a series of failed reproductive seasons (57). Species with high biomass values in Denmark thus may exhibit enhanced abilities to colonize and persist in suitable patches of habitat. The total breeding avifauna is estimated at 1.643 × 107 pairs ranging from <10 pairs (27 species) to 2,228,000 pairs (Turdus merula) per species. The three most abundant species (Alauda arvensis, Turdus merula, and Fringilla coelebs) constituted 32.5% of the total individuals, but 71 species (36%) had breeding populations >10,000 pairs (Table S2). Narrowly distributed species exhibit a strong range size–abundance relationship, but the correlation is weaker for geographically widespread species in Denmark (58). Estimates of Danish population biomass ranged from <100 g (seven species) to 6.3 × 108 g (Phasianus colchicus).
Analysis of Ecological Guilds.
We categorized the Danish breeding avifauna into two types of ecological guilds. First, we grouped 194 of 197 species into 33 mutually exclusive foraging guilds, which pool mixtures of congeneric and more distantly related species that use a similar spectrum of resources. We also analyzed a subset of eight narrowly defined congeneric guilds composed of closely related species (Table S2). To maintain statistical power in guild analyses, we focused on guilds that contained four or more species (171 species in 24 foraging guilds, and 40 species in eight congeneric guilds). For all analyses, the spatial domain included only those cells that contained at least one guild member. This restriction guards against spurious patterns of aggregation that might arise from including empty cells that are not biologically suitable for any of the species in the guild.
Quantification of Species Co-Occurrence Patterns.
We used the C-score (59) as a quantitative index of species co-occurrence. The C-score is defined as (Ri – S) × (Rj – S) where Ri and Rj represent the total number of occurrences of species i and j, respectively, and S is the number of shared occurrences. The average C-score, calculated over all unique species pairs within an ecological guild, summarizes the pattern of co-occurrence as a single metric. The larger the C-score, the fewer incidents of co-occurrence among pairs of species. However, the C-score, like most indices of segregation or aggregation, is affected both by the number of shared occurrences and by the total number of occurrences of each species. For this reason, comparison with an appropriate suite of null models is essential.
Randomization Tests.
We compared the C-score observed for ecological guilds of breeding birds with scores generated by four different null models ranging in complexity from a simple constrained randomization of the binary presence/absence matrix to models that incorporated measures of habitat heterogeneity, population size, and biomass (for model details, SI Text). For each model, we created null avifaunal assemblages (n = 1,000) and calculated the C-score for each. We then compared the C-score observed for ecological guilds with the distribution of simulated C-scores to estimate the one-tailed probability. Each set of simulations was initialized with a new random number seed taken from the system clock, and all null-model analyses were conducted in EcoSim Version 7.2 (60).
Analyses of Habitat Niche Overlap.
We used a null model based on the “habitat utilization matrix” (33) to determine whether species’ co-occurrence patterns were associated with the coarse-grained distribution of habitats. For each species, we determined the total area of each of the 12 habitat categories in cells that it occupied. We then constructed a habitat utilization matrix in which each row represents a species, each column represents a habitat category, and the entries are the summed areas of the habitat categories in each occupied cell.
The habitat areas then were converted to percentages for each species. For each unique species pair ij, we calculated habitat niche overlap Oij using Pianka’s (31) overlap index as:
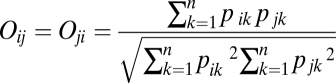 |
where pik is the proportional occupancy of cells containing habitat k by species i. If Oij = 0.0, then species i and j occur in cells that do not share any habitat categories. In contrast, high index values indicate that species occur in cells that contain similar proportions of the various habitat categories. We then calculated the average pairwise overlap for all unique species pairs in the matrix. Habitat utilization matrices were calculated at both spatial scales for foraging and congeneric guilds. Note that the spatial scales of our analyses are relatively large compared with the scale at which avian habitat selection occurs. The metrics describe overlap in the habitat distributions of occupied sites, which is not necessarily identical with overlap in habitat utilization.
We compared the average pairwise overlap in real assemblages of species with the frequency distribution of overlap values observed in null assemblages. The null distribution was created by reshuffling the overlap values within each row of the original species × habitat utilization matrix to generate a null distribution (1,000 randomizations) that would be expected if habitat utilization was independent among species. We then calculated the probability that the observed niche overlap was drawn from this distribution (61).
One potential problem with such niche overlap analyses is that they assume that all of the resource states, or in this case habitat categories, are equally abundant (62). This assumption is not met for the habitats of Denmark, which vary considerably in their total area. We therefore analyzed “electivity indices” of species by dividing the observed utilization values for each habitat category by the total area of that habitat in Denmark (32, 63). This scaling gives less weight to common habitats, which will tend to dominate the numerical results in the unweighted analysis.
Supplementary Material
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0914089107/DCSupplemental.
References
- 1.Gleason HA. The individualistic concept of the plant association. Bull Torrey Bot Club. 1926;53:7–26. [Google Scholar]
- 2.Cody ML, Diamond JM, editors. Ecology and Evolution of Communities. Cambridge, MA: Harvard Univ Press; 1975. [Google Scholar]
- 3.Weiher E, Keddy P, editors. Ecological Assembly Rules: Perspectives, Advances, Retreats. Cambridge, U.K.: Cambridge Univ Press; 1999. [Google Scholar]
- 4.Ricklefs RE, Schluter D, editors. Species Diversity in Ecological Communities. Chicago: Univ of Chicago; 1993. [Google Scholar]
- 5.Choler P, Michalet R, Callaway RM. Facilitation and competition on gradients in alpine plant communities. Ecology. 2001;82:3295–3308. [Google Scholar]
- 6.Goldberg DE, Barton AM. Patterns and consequences of interspecific competition in natural communities—a review of field experiments with plants. Am Nat. 1992;139:771–801. [Google Scholar]
- 7.Kurle CM, Croll DA, Tershy BR. Introduced rats indirectly change marine rocky intertidal communities from algae- to invertebrate-dominated. Proc Natl Acad Sci USA. 2008;105:3800–3804. doi: 10.1073/pnas.0800570105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paine RT. Marine Rocky Shores and Community Ecology: An Experimentalist’s Perspective. Germany: Ecology Institute, Oldendorf/Luhe; 1994. [Google Scholar]
- 9.Wootton JT. Markov chain models predict the consequences of experimental extinctions. Ecol Lett. 2004;7:653–660. [Google Scholar]
- 10.Sanders NJ, Gordon DM. Resource-dependent interactions and the organization of desert ant communities. Ecology. 2003;84:1024–1031. [Google Scholar]
- 11.Munday PL, Jones GP, Caley MJ. Interspecific competition and coexistence in a guild of coral-dwelling fishes. Ecology. 2001;82:2177–2189. [Google Scholar]
- 12.Schoener TW, Losos JB, Spiller DA. Island biogeography of populations: An introduced species transforms survival patterns. Science. 2005;310:1807–1809. doi: 10.1126/science.1120165. [DOI] [PubMed] [Google Scholar]
- 13.Meserve PL, Milstead WB, Gutierrez JR. Results of a food addition experiment in a north-central Chile small mammal assemblage: Evidence for the role of “bottom-up” factors. Oikos. 2001;94:548–556. [Google Scholar]
- 14.Robinson SK, Terborgh J. Interspecific aggression and habitat selection by Amazonian birds. J Anim Ecol. 1995;64:1–11. [Google Scholar]
- 15.Orians GH, Willson MF. Interspecific territories of birds. Ecology. 1964;45:736–745. [Google Scholar]
- 16.Cody ML. Competition and the Structure of Bird Communities. Princeton, NJ: Princeton Univ Press; 1974. [PubMed] [Google Scholar]
- 17.Schluter D, Grant PR. The distribution of Geospiza difficilis in reliation to Geospiza fulignosa in the Galapagos islands—tests of three hypotheses. Evolution. 1982;36:1213–1226. doi: 10.1111/j.1558-5646.1982.tb05490.x. [DOI] [PubMed] [Google Scholar]
- 18.MacArthur RH, Diamond JM, Karr J. Density compensation in island avifaunas. Ecology. 1972;53:330–342. [Google Scholar]
- 19.Crowell KL. Reduced interspecific competition among the birds of Bermuda. Ecology. 1962;43:75–88. [Google Scholar]
- 20.Diamond JM. In: Ecology and Evolution of Communities. Cody ML, Diamond JM, editors. Cambridge, MA: Harvard Univ Press; 1975. pp. 342–444. [Google Scholar]
- 21.Catchpole CK. Interspecific territorialism and competition in Acrocephalus warblers as revealed by playback experiments in areas of sympatry and allopatry. Anim Behav. 1978;26:1072–1080. [Google Scholar]
- 22.Connor EF, Simberloff D. The assembly of species communities: Chance or competition? Ecology. 1979;60:1132–1140. [Google Scholar]
- 23.Gotelli NJ, Buckley NJ, Wiens JA. Co-occurrence of Australian land birds: Diamond’s assembly rules revisited. Oikos. 1997;80:311–324. [Google Scholar]
- 24.Wiens JA. The Ecology of Bird Communities. Cambridge, MA: Cambridge Univ Press; 1989. [Google Scholar]
- 25.Cody ML. Competition and the Structure of Bird Communities. San Diego, California: Academic.; 1985. [PubMed] [Google Scholar]
- 26.Murray BG. The origins of adaptive interspecific territorialism. Biol Rev Camb Philos Soc. 1981;56:1–22. [Google Scholar]
- 27.MacArthur RH. Geographical Ecology. New York: Harper and Row; 1972. [Google Scholar]
- 28.Sluiters JE. Observations on the Kentish, Little-Ringed and Ringed Plover, breeding near Amsterdam. Limosa. 1954;27:71–86. [Google Scholar]
- 29.Connor EF, Bowers MA. The spatial consequences of interspecific competition. Ann Zool Fenn. 1987;24:213–226. [Google Scholar]
- 30.Bønløkke J, et al. Dansk Trækfugleatlas. Humlebæk, Denmark: Rhodos; 2006. [Google Scholar]
- 31.Pianka ER. Niche overlap and diffuse competition. Proc Natl Acad Sci USA. 1974;71:2141–2145. doi: 10.1073/pnas.71.5.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schoener TW. Some methods for calculating competition coefficients from resource-utilization spectra. Am Nat. 1974;108:332–340. doi: 10.1086/282911. [DOI] [PubMed] [Google Scholar]
- 33.Fretwell SD, Lucas HL. On territorial behavior and other factors influencing habitat distribution in birds: I. Theoretical development. Acta Biotheor. 1969;19:16–36. [Google Scholar]
- 34.Stamps JA. The effects of conspecifics on habitat selection in territorial species. Behav Ecol Sociobiol. 1991;28:29–36. [Google Scholar]
- 35.Danchin E, Wagner RH. The evolution of coloniality: The emergence of new perspectives. Trends Ecol Evol. 1997;12:342–347. doi: 10.1016/s0169-5347(97)01124-5. [DOI] [PubMed] [Google Scholar]
- 36.Forsman JT, Mönkkönen M, Helle P, Inkeröinen J. Heterospecific attraction and food resources in migrants’ breeding patch selection in northern boreal forest. Oecologia. 1998;115:278–286. doi: 10.1007/s004420050517. [DOI] [PubMed] [Google Scholar]
- 37.Stephens PA, Sutherland WJ, Freckleton RP. What is the Allee effect? Oikos. 1999;87:185–190. [Google Scholar]
- 38.Stephens PA, Sutherland WJ. Consequences of the Allee effect for behaviour, ecology and conservation. Trends Ecol Evol. 1999;14:401–405. doi: 10.1016/s0169-5347(99)01684-5. [DOI] [PubMed] [Google Scholar]
- 39.Cody ML. Habitat selection and interspecific territoriality among the sylviid warblers of England and Sweden. Ecol Monogr. 1978;48:351–396. [Google Scholar]
- 40.Bourski OV, Forstmeier W. Does interspecific competition affect territorial distribution of birds? A long-term study on Siberian Phylloscopus warblers. Oikos. 2000;88:341–350. [Google Scholar]
- 41.Hoi H, Eichler T, Dittami J. Territorial spacing and interspecific competition in 3 species of reed warblers. Oecologia. 1991;87:443–448. doi: 10.1007/BF00634604. [DOI] [PubMed] [Google Scholar]
- 42.Reed TM. Interspecific territoriality in the Chaffinch and Great Tit on islands and the mainland of Scotland—playback and removal experiments. Anim Behav. 1982;30:171–181. [Google Scholar]
- 43.Grinnell J. The niche-relationships of the California thrasher. Am Nat. 1917;51:115–128. [Google Scholar]
- 44.Lack D. Habitat selection in birds. With special reference to the effects of afforestation on the Breckland avifauna. J Anim Ecol. 1933;2:239–262. [Google Scholar]
- 45.James FC, Johnston RF, Wamer NG, Niemi GJ, Boecklen WJ. The Grinnelian niche of the wood thrush. Am Nat. 1984;124:17–30. [Google Scholar]
- 46.Hinsley SA, Bellamy PE, Newton I, Sparks TH. Habitat and landscape factors influencing the presence of individual breeding bird species in woodland fragments. J Avian Biol. 1995;26:94–104. [Google Scholar]
- 47.Araujo MB, Rahbek C. How does climate change affect biodiversity? Science. 2006;313:1396–1397. doi: 10.1126/science.1131758. [DOI] [PubMed] [Google Scholar]
- 48.Araujo MB, Luoto M. The importance of biotic interactions for modelling species distributions under climate change. Glob Ecol Biogeogr. 2007;16:743–753. [Google Scholar]
- 49.Lagerlund E, Houmark-Nielsen M. Timing and pattern fo the last deglaciation in the Kattegat region, southwest Scandinavia. Boreas. 1993;22:337–347. [Google Scholar]
- 50.Aaris-Sørensen K. The Holocene history of the Scandinavian aurochs (Bos primigenius Bojanus, 1827) Wissenschaftliche Schriften des Neanderthal Museums. 1999;1:49–57. [Google Scholar]
- 51.Grell MB. Fuglenes Danmark. Copenhagen, Denmark: Gads Forlag; 1998. [Google Scholar]
- 52.Vestergaard P, editor. Naturen i Danmark: Det åbne land. Copenhagen, Denmark: Gyldendal; 2007. [Google Scholar]
- 53.Groom G, Stjernholm M. In: Strategic Landscape Monitoring for the Nordic Countries. Groom G, Reed T, editors. Copenhagen, Denmark: Nordic Council of Ministers; 2001. pp. 81–87. [Google Scholar]
- 54.Hurlbert SH. The nonconcept of species diversity: A critique and alternative parameters. Ecology. 1971;52:577–585. doi: 10.2307/1934145. [DOI] [PubMed] [Google Scholar]
- 55.MacArthur RH, Wilson EO. The Theory of Island Biogeography. Princeton, NJ: Princeton Univ Press; 1967. [Google Scholar]
- 56.Bonner JT. Size and Cycle: An Essay on the Structure of Biology. Princeton, NJ: Princeton Univ Press; 1965. [Google Scholar]
- 57.Tracy CR, George TL. On the determinants of extinction. Am Nat. 1992;139:102–122. [Google Scholar]
- 58.Borregaard MK, Rahbek C. Prevalence of intraspecific relationships between range size and abundance in Danish birds. Divers Distrib. 2006;12:417–422. [Google Scholar]
- 59.Stone L, Roberts A. The checkerboard score and species distributions. Oecologia. 1990;85:74–79. doi: 10.1007/BF00317345. [DOI] [PubMed] [Google Scholar]
- 60.Gotelli NJ, Entsminger GL. EcoSim: Null models software for ecology. Version 7. (Acquired Intelligence & Kesey-Bear Inc) 2004. http://garyentsminger.com/ecosim.htm.
- 61.Winemiller KO, Pianka ER. Organization in natural assemblages of desert lizards and tropical fishes. Ecol Monogr. 1990;60:27–55. [Google Scholar]
- 62.Gotelli NJ, Graves GR. Null Models in Ecology. Washington, D.C.: Smithsonian Institution Press; 1996. [Google Scholar]
- 63.Lawlor LR. Overlap, similarity, and competition coefficients. Ecology. 1980;61:245–251. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.