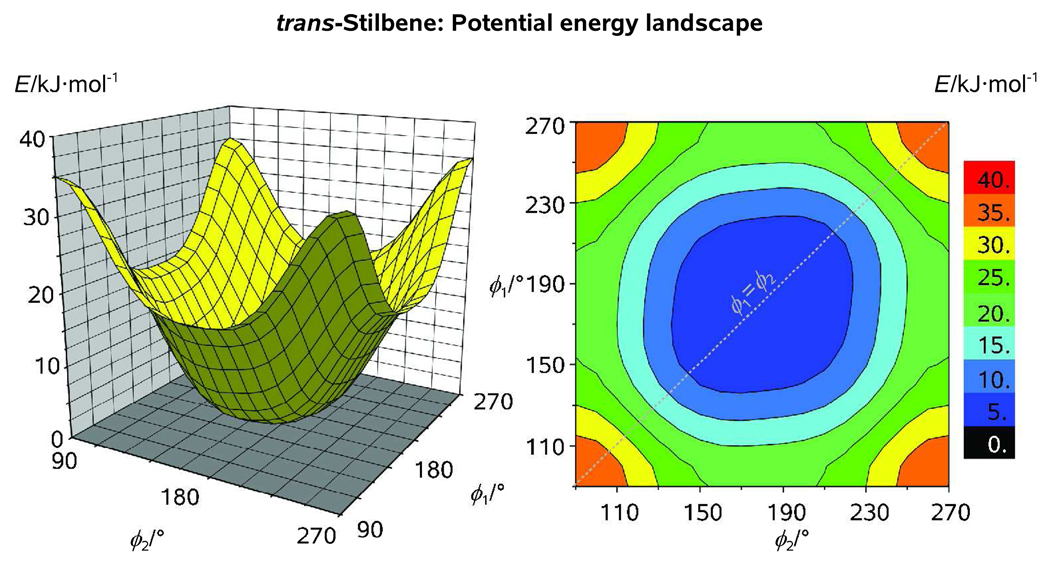
Figure 6.
Rotational potential of trans-stilbene as obtained from a relaxed (ϕ1, ϕ2) scan calculation with a step of 10° at our standard computational level; Note that, despite the flatness of the potential in the vicinity of the equilibrium structure, there exists a slight bias toward the nonplanar pseudoconformers of trans-stilbene which possess a quasi-C2 symmetry (ϕ1 ≈ ϕ2).