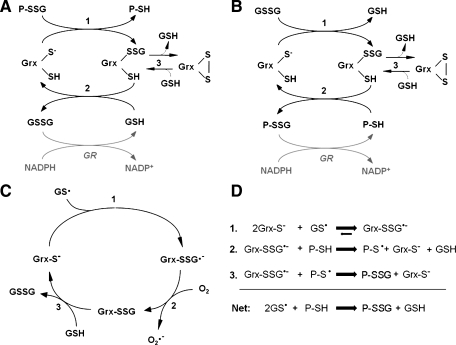
FIG. 3.
(A) Catalytic mechanism of deglutathionylation by human glutaredoxins (38, 47, 122). In the first step, the thiolate of the Grx N-terminal active-site cysteine attacks the glutathionyl sulfur of the protein-glutathione mixed disulfide (P-SSG), forming the Grx-SSG intermediate and releasing reduced protein-SH (P-SH). In the second step, free GSH attacks the glutathionyl sulfur of the Grx-SSG intermediate, releasing reduced Grx and GSSG. GSSG is then reduced to 2GSH by GSSG reductase (GR) and NADPH. Step 3 represents a side reaction in which the Grx C-terminal active-site cysteine competes with GSH for reduction of Grx-SSG, forming a Grx active-site disulfide and releasing GSH. The Grx-S2 side product is reduced by GSH and recruited back into the catalytic cycle. (B) Grx can use GSSG as an oxidized substrate (38, 83, 124). This scheme is analogous to Scheme 1A, except that the first substrate for Grx is glutathionylated glutathione (i.e., GSSG), and the second substrate is protein-SH. This reaction occurs under oxidizing conditions (i.e., low GSH/GSSG ratio) until the protein-SH/protein-SSG ratio reaches equilibrium. (C) Proposed mechanism of glutathione thiyl radical (GS•) scavenging by Grx (124). In the first step, the N-terminal active-site cysteine of Grx attacks GS•, forming a Grx disulfide anion radical intermediate. This radical then reacts with O2 in Step 2, forming superoxide (O2•−) and the typical Grx-SSG intermediate. In Step 3, the Grx-SSG intermediate is reduced by GSH, forming GSSG and reduced enzyme. (D) Proposed mechanism of glutathionyl transfer by Grx (124). In the first step of the reaction, the Grx catalytic cysteine thiolate attacks GS•, forming the Grx-SSG•− disulfide anion radical intermediate. This intermediate can proceed to react with protein-SH (P-SH, Step 2), forming protein thiyl radical (P-S•). In Step 3, another Grx-SSG•− molecule reacts with P-S• (Step 3), quenching the radical reaction and forming protein-SSG (P-SSG). The net reaction yields protein-SSG from two GS• and two P-SH molecules.