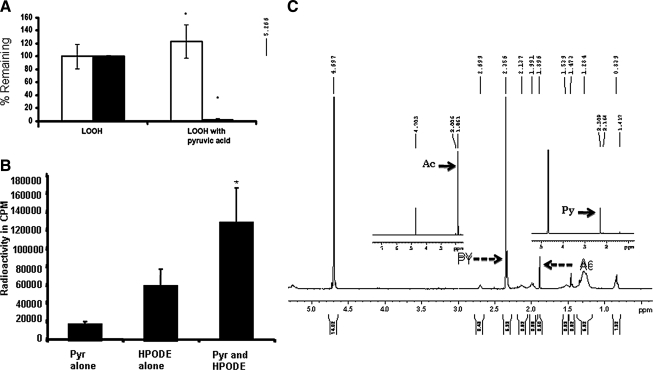
FIG. 6.
(A) Reaction between lipid hydroperoxide and pyruvic acid. The 500 nmols/ml of 13-HPODE was reacted with 7.5 μmols/ml of PYR in 1 ml of PBS at pH 7.4 for 5 min at room temperature. After the incubation, a 50-μl aliquot of the sample (in duplicate) was taken for the LMB assay, as described in Methods. The remaining sample was acidified with 25 μl of 1N HCl and extracted with 2 ml of ether. Ether was then evaporated overnight, and the resultant residue was dissolved in methanol. The conjugated diene was measured by reading the spectrum at 234 nm, with methanol as blank. The experiment was done in triplicate. The graph is an average of three experiments done with fresh LOOH and PYR and expressed as a percentage of the remaining diene and peroxide. The Y axis represents the percentage of the remaining diene and peroxide in the reaction; this is statically significant (p < 0.05). (B) CO2 trapping in lipid hydroperoxide and pyruvic acid reaction: the 50 μM LOOH reacted with a similar concentration of PYR in a microtube. The mixture was incubated at 37°C in a dry-bath incubator. The carbon dioxide was trapped by using filter paper, as previously described in Methods. The Y axis represents the percentage of radioactive calcium carbonate present in the filter paper. Formation of calcium carbonate and rate of CO2 released was significant (p < 0.05). (C) NMR spectroscopy of the reaction between pyruvate and 13-HPODE. The 12 μmoles of HPODE was allowed to react with 60 μmoles of PYR at room temperature. After overnight incubation, the samples were lyophilized and dissolved in 750 μl deuterium oxide and measured in 400-MHz 1H-NMR. Inserts are spectra of acetate and pyruvate for comparison.