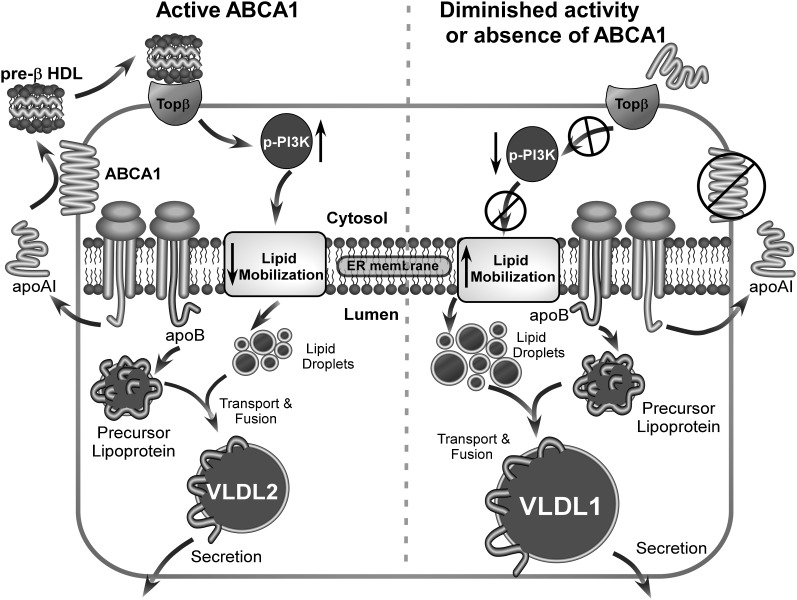
Fig. 8.
Summary diagram of experimental results. In the presence of active ABCA1 (left), lipid free apoA-I is secreted by hepatocytes and lipidated by hepatocyte ABCA1, forming nascent HDL particles. Large nascent HDLs (>10 nm diameter) bind to a putative receptor (Topβ, target of pre-β), which in turn stimulates PI3 kinase activation and results in reduced lipid mobilization and secretion of normal-sized VLDL particles (i.e., VLDL2). In contrast, in the absence of hepatic ABCA1 or diminished ABCA1 activity (right), newly secreted lipid-free apoA-I fails to form large nascent HDL particles. The absence of large nascent HDL particle formation leads to diminished signaling through Topβ, resulting in reduced PI3 kinase activation, increased lipid mobilization, and increased secretion of larger TG-enriched VLDL particles (VLDL1).