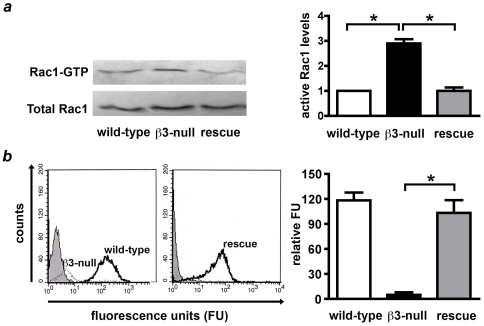
Figure 1. Increased active levels of Rac1 in β3-integrin-deficient endothelial cells.
A. Active levels of Rac1 were examined by GST-PAK pull-downs. Western blot analysis of active Rac1 bound to GST-PAK (Rac1-GTP) and total Rac1 from wild-type, β3-integrin null, and β3-null primary lung endothelial cells transduced with human β3-integrin (rescue). Immunoblots were quantified by densitometry, and the levels of GTP-bound Rac1 normalised to total Rac1 levels. Active levels of Rac1 were increased approximately 3 fold in β3-null endothelial cells when compared with wild-type controls (*P<0.01), however, total levels of Rac1 were expressed equally in both genotypes. Furthermore, active levels of Rac1 were reduced to wild-type levels in rescue cells (*P<0.01). Results shown are the means + s.e.m of 3-4 independent experiments. B. Flow-cytometric analysis shows that surface levels of β3-integrin were not detectable in β3-null (dashed line) cells when compared with wild-types (bold line) endothelial cells (left panel). Rescue cells expressed β3-integrin (bold line, right panel). Grey peaks represent isotope IgG controls. Bar graph shows means + s.e.m of relative surface β3-integrin expression in wild-type (white), β3-null (black) and rescue (grey) endothelial cells compared with negative control (*P<0.001, N = 3 independent experiments).