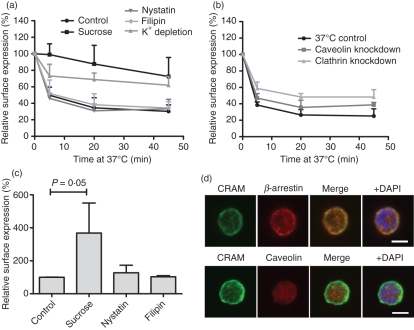
Figure 5.
Endocytosis of chemokine receptor on activated macrophages (CRAM) is clathrin dependent in Nalm6 cells. (a) The effect of different endocytosis inhibitors on CRAM internalization was studied in Nalm6 cells. Cells were pre-incubated at 37° with the indicated inhibitors of clathrin-coated pits or caveolae-mediated pathways for 1 hr before performing an internalization assay as in Fig. 4 (t test P values at 20 min are 0·0017, 0·0019, 0·67 and 0·69 for sucrose, K+ depletion, nystatin and filipin treatments, respectively). Data shown are the mean and range of three independent experiments. (b) The effect of small interfering RNA down-regulation of clathrin and cavelin-1 was assessed in the same conditions. Data show mean and SD from three independent experiments (t-test P values are 0·0148, 0·0081, 0·0320 for clathrin knockdown at 5, 20 and 45 min, respectivelyl caveolin knockdown induced only insignificant changes in internalization rate). (c) Surface expression levels of CRAM were assessed by fluorescence activated cell sorting staining after a 1-hr incubation with different endocytosis inhibitors. (d) Immunofluorescence analysis of fixed, permeabilized Nalm6 cells stained with anti-CRAM and alexa488 coupled secondary antibody (green) shows co-localization of CRAM when stained for β-arrestin (top panels), but not when stained for caveolin (bottom panels). Both antibodies were recognized by an Alexa647 anti-rabbit secondary antibody (red). The nucleus was revealed using DAPI (blue). White bars depict 5 μm.