Abstract
Viral infections of the pediatric central nervous system (CNS) encompass a broad spectrum of both perinatally and postnatally acquired diseases with potentially devastating effects on the developing brain. In children, viral infections have been associated with chronic encephalopathy, encephalitis, demyelinating disease, tumors, and epilepsy. Older diagnostic techniques of biopsy, viral culture, electron microscopy, gel-based polymerase chain reaction (PCR), and viral titer quantification are being replaced with more rapid, sensitive, and specific real-time and microarray-based PCR technologies. Advances in neuroimaging technologies have provided for earlier recognition of CNS injury without elucidation of specific viral etiology. Although the mainstay therapy of many pediatric neurovirologic diseases, aside from HIV, includes intravenous acyclovir, much work is being done to develop novel antiviral immunotherapies aimed at both treating and preventing pediatric CNS viral disease.
Keywords: pediatric neurovirology, CNS
Introduction
Pediatric neurovirology, although not a formally recognized subspecialty, is a collaborative effort among many disciplines, including neonatology, infectious disease, immunology, neurology, neuropathology, and neuroradiology. Central nervous system (CNS) viral infections pose a particular problem in the pediatric population, because both perinatally and postnatally acquired disease may lead to devastating consequences for the developing nervous system. For example, congenital human cytomegalovirus (CMV) infection, occurring in up to 0.7% of newborns, causes microcephaly, cerebral palsy, developmental delay/mental retardation, and sensorineural hearing and vision loss [1]. Neonatal herpes simplex virus types 1 and 2 (HSV-1, HSV-2) invade the CNS in approximately 1500 newborns in the United States yearly, and may occur via intrauterine (5% of cases), perinatal (85% of cases), or postnatal (5% of cases) routes, often with neurologic outcomes similar to those of CMV [2]. In post-term infants and children, viral encephalitis remains a considerable diagnostic dilemma for clinicians. Despite modern diagnostic techniques, a specific viral etiology could be assigned in only 9% to 30% of cases in three large multicenter prospective encephalitis studies worldwide [3–5]. CNS infection with human herpesvirus 6 (HHV-6), a ubiquitous beta herpesvirus that causes roseola infantum, is increasingly recognized as a potential culprit of both pediatric encephalitis [6] and epilepsy [7]. The role of neuroimaging in pediatric and adult CNS infectious disease is playing an increasingly larger role in diagnosing encephalitis [8]. Through correlation of MRI findings with laboratory results, it is possible to generate a reasonable differential diagnosis based on published associations; however, this approach remains considerably insensitive. One major limitation in associating viral infections with disease remains the sensitivity and specificity of the many molecular diagnostic techniques currently used [9]. Aside from government-funded agencies such as the California Encephalitis Project [3], which performs a multitude of standardized and sophisticated virologic assays, many clinicians often order a potpourri of diagnostic tests that are either largely incomplete or excessive, often without diagnostic yield. Neurotropic viruses, both known and emerging, are being studied in association with a variety of pediatric diseases, including encephalitis, demyelinating diseases, tumors, chronic encephalopathy, and epilepsy, to name a few. The design of more sophisticated diagnostic modalities and specific viral therapies is at the forefront of pediatric neurovirology research.
Molecular Diagnostic Testing of Pediatric CNS Viral Disease
Before the advent of the polymerase chain reaction (PCR) assay, diagnostic testing in neurovirology was performed mostly by means of biopsy, viral culture, and antibody titer analysis. One of the difficulties with viral culture analysis is that it may take between 1 and 28 days for results, with poor sensitivity. Viral titer analysis has a similar problem of cumbersomeness (requiring up to 4 weeks for a convalescent phase rise in titers), along with a high seroprevalence of many neurotropic viruses, limiting the overall specificity of the assay. Despite these limitations, elevations of some cerebrospinal fluid (CSF) viral titers (West Nile virus, St. Louis encephalitis virus, varicella zoster virus [VZV]) are diagnostic. In the cases of the Flaviviridae and Togaviridae virus families, acute and convalescent titer elevations remain diagnostic [10••]. PCR has revolutionized molecular-based testing in neurovirology and has since evolved into several varieties. PCR amplification techniques currently used for viral nucleic acid detection in pediatric CNS disease include conventional PCR, nested PCR, real-time PCR (RT-PCR), multiplex PCR, and more recently, loop-mediated isothermal amplification (LAMP) assay. In patients with presumed encephalitis, nucleic acids are extracted from CSF with the premise that active viral infections in the brain will exhibit maximum viral nucleic acid production, reflected in the CSF [9]. Conventional qualitative PCR amplifies millions of copies of template nucleic acid (from 100–200 µL of CSF per PCR reaction) with just 1 to 10 target copies of DNA/RNA in less than 24 h of processing through the use of DNA-based primers, a heat-sensitive DNA polymerase, a thermocycler, and a gel-based detection system. The validity of conventional PCR was best studied in a large series of biopsy-proven herpesvirus encephalitis cases, in which HSV PCR was 96% specific and 99% sensitive [11]. Nested PCR uses a double amplification of template DNA with a set of inner and outer primers to achieve a sensitivity higher than that of conventional PCR; however, it also is more time consuming and more prone to contamination. Recently, a nested PCR assay was designed to detect aerosolized respiratory viruses, including influenza A/B, parainfluenza, and human respiratory syncytial virus, to aid in rapid diagnosis [12]. RT-PCR, the current standard of care in most diagnostic laboratories, uses a fluorescent probe–based technology with a fully automated detection system to more accurately quantitate starting template DNA through real-time analysis of DNA amplification. RT-PCR is particularly useful in characterizing viruses of known latency (eg, Epstein-Barr virus [EBV]) or following trends of viral nucleic acids after therapy. More recently, LAMP PCR technology was designed to enhance the detection limits by 10- to 100-fold without the need for sophisticated equipment [13]. LAMP involves the design of six primers that bind to eight regions on the target DNA to form stem–loop structures through both cyclic and noncyclic amplification steps. The amplified products can be monitored quantitatively in real time by either visual turbidity or fluorescence and currently are used to detect a variety of known and emerging neuropathogens [14]. To circumvent the problem of the relatively small amounts of pediatric CSF obtained during lumbar puncture, two multiplex PCR assays for detecting herpesviruses in pediatric patients were reported recently [15, 16]. Multiplex PCR uses one reaction tube with a multitude of virus-specific primers to yield different size products depending on the target viral sequence. Wada et al. [15] used unique fluorochrome-linked probes for each of four herpesvirus primers (HSV-1, HSV-2, HHV-6, and HHV-7), with a reported sensitivity of 4 copies/reaction. Shi et al. [16] used a similar multiplex PCR assay for several herpesviruses (HSV-1, HSV-2, EBV, CMV, and HHV-6) in pediatric patients, except they conjugated the amplified PCR products to a fluorochrome and hybridized them onto a glass chip containing a set of virus-specific DNA sequences. Compared with standard RT-PCR assays, Shi et al. [16] reported 91.7% sensitivity and 100% specificity. These emerging technologies most likely will pave the way for comprehensive one-step multiplex PCR assays that will use “viral DNA chips” of the more than 100 viruses known to be associated with human encephalitis and other CNS diseases.
Role of Neuroimaging in Pediatric Viral Encephalitis
MRI increasingly is regarded as an important adjuvant tool in the diagnosis and surveillance of pediatric neurovirologic disease. Neuroimaging largely has been used retrospectively in pediatric neurovirology upon laboratory confirmation of disease. The many published case reports of confirmed viral encephalitides with associated MRI abnormalities have served as the initial neuroatlas of pediatric neurovirology, and MRI remains one of the most sensitive tests for diagnosing encephalitis in children [8, 17–24]. Aside from the more typical findings of temporal/inferior frontal abnormalities seen on MRI in HSV encephalitis or subependymal gadolinium enhancement in CMV encephalitis, there remains considerable overlap among neurotropic viruses in both appearance and location on MRI imaging, as represented in Fig. 1. Japanese encephalitis (JE), a zoonotic RNA flavivirus endemic to Asia that accounts for 10,000 deaths per year, has been among the most widely studied in terms of neuroimaging features, largely because of its high prevalence. Dung et al. [17] found that thalamic edema was the most frequent abnormality on CT/MRI in 10 of 43 CSF antibody-confirmed pediatric JE cases, with a negative predictive value of 42.1% and a sensitivity of 100%. However, the specificity of bithalamic edema on neuroimaging also has been reported in association with influenza [18] and HHV-6 necrotizing encephalitis [19, 20], among others. In addition to thalamic abnormalities, West Nile virus, a flavivirus similar to JE, has been associated in a small subset of patients with MRI changes in the basal ganglia, brainstem, cerebellum, periventricular white matter, mesial temporal lobe, and anterior horns of the spinal cord similar to those seen in poliomyelitis [21]. Diffusion-weighted imaging (DWI) and apparent diffusion coefficient (ADC) mapping may be useful adjuvant MRI sequences in detecting early signs of viral-mediated vasogenic edema. Compared with JE patients, HSV encephalitis patients showed significantly more restricted diffusion and lower ADC signal in the acute than in the chronic stage of infection, which may be one of the earliest detectable signs in HSV encephalitis [22]. DWI frontal lobe abnormalities also have been reported in patients with HHV-6 encephalitis [19, 23], in addition to the other signal changes reported in HHV-6 encephalitis involving the hippocampus, extrahippocampal structures, and rhombencephalon [19, 20, 23, 24], which often lead to chronic necrotization [20]. Single photon emission CT, magnetic resonance spectroscopy, and diffusion tensor imaging have been studied far less in pediatric viral encephalitis and currently have no proven diagnostic or prognostic value. Advances in molecular diagnostics, together with improved neuroimaging techniques, will have to work in tandem to help solve the many challenges clinicians face in establishing a neurovirologic diagnosis.
Fig. 1.
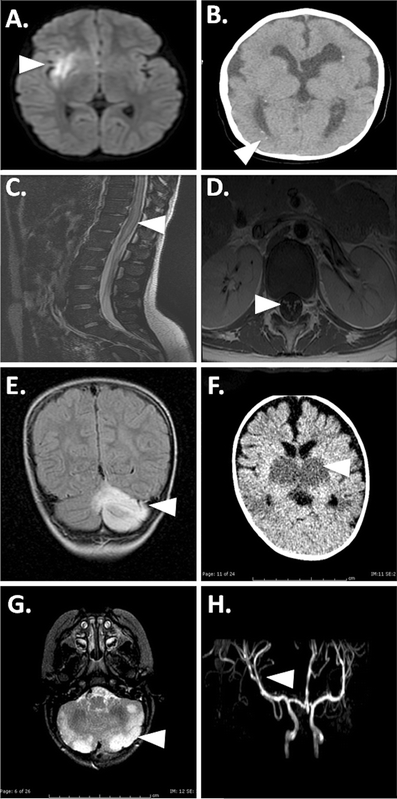
MRI findings in viral-associated pediatric CNS disease. a Increased diffusion-weighted imaging signal in the right mesial temporal lobe (arrowhead) in a patient with polymerase chain reaction (PCR)-confirmed herpes simplex virus (HSV)-1 encephalitis. b Periventricular calcifications (arrowhead), diffuse volume loss, and cerebral dysgenesis in an infant with antibody-positive congenital cytomegalovirus. c Transverse myelitis (arrowhead) secondary to HSV-2 in a sexually active teenager with HSV-2–positive cerebrospinal fluid (CSF) PCR and cervical culture. d West Nile virus confirmed by CSF serology in a child with acute lower-extremity paresis revealed by anterior ventral nerve root enhancement on MRI (arrowhead). e Rapidly progressive encephalopathy with cerebellar ataxia in a patient with human herpesvirus (HHV)-6 encephalitis and abnormal cerebellar signal on MRI (arrowhead) confirmed by HHV-6 CSF and biopsy-positive PCR. f Necrotizing encephalopathy in an infant with influenza B revealed by bithalamic hyperdensities on CT scan (arrowhead). g Acute encephalopathy in an infant with CSF-positive respiratory syncytial virus PCR revealed by diffuse cerebellar abnormalities (arrowhead). h Varicella zoster virus vasculitis in a patient during acute varicella infection manifested in a right basal ganglia infarct secondary to right middle cerebellar artery narrowing (arrowhead)
Treatment of Pediatric CNS Neurovirologic Disease
Although the current treatment for many pediatric CNS neurovirologic diseases, exclusive of HIV, is largely supportive, antiviral therapy remains the treatment of choice for infections involving the Herpesviridae family of viruses. The overwhelming mechanism of action commonly evoked by antiviral therapy involves inhibition of viral DNA polymerase. In the case of acyclovir, an acyclic deoxyguanosine analogue, three phosphorylations are required (one by a viral thymidine kinase) before activation to competitively inhibit viral DNA polymerase and intercalate into newly synthesized viral DNA to terminate viral replication. Consequently, antiviral therapy works most efficiently during early viral replication. Acyclovir therapy has had tremendous success in improving survival in neonatal HSV encephalitis, from 50% in the pre-acyclovir era to 14% with the standard dosage (30 mg/kg per day divided three times daily) and 4% with the high dosage (60 mg/kg per day divided three times daily) [25]. Unfortunately, acyclovir therapy has not had any effect on neurodevelopmental outcomes in neonates compared with untreated patients (67% vs 69%) [25]. By comparison, in the postneonatal period, acyclovir has improved both mortality (70% vs 28%) and neurologic morbidity (97% vs 62%) at the standard 21-day dosage of 30 mg/kg per day divided three times daily [2]. Based on these findings, the Infectious Diseases Society of America recommends treating all patients with suspected encephalitis with acyclovir until HSV encephalitis has been ruled out [10••]. In terms of treatment duration, 21 days is generally recommended, although HSV PCR will remain positive in a subset of patients, possibly warranting prolonged therapy [10••]. On the other hand, up to 13% of pediatric patients have been shown to be HSV PCR negative early in the disease course [26]; hence, it is recommended that when there is clinical and/or radiographic suspicion of HSV encephalitis, repeat HSV PCR be performed within 3 to 7 days (category B evidence) [10••]. If acyclovir is not available—as was the case in early 2009, when the US Food and Drug Administration reported a shortage of acyclovir from the manufacturer—ganciclovir or foscarnet is a reasonable alternative; however, both agents have substantial renal and hematologic side effects relative to acyclovir. Aside from HSV encephalitis treatment with acyclovir, treatment of the other CNS herpesvirus-associated diseases carries a lower level of recommendation based on the lack of available evidence [10••]. In the case of congenital CMV, ganciclovir at 12 mg/kg per day divided twice daily for 6 weeks has improved hearing outcomes [25]. A subsequent phase 3 randomized trial in 100 neonates randomly assigned to receive a 6-week course of ganciclovir therapy (12 mg/kg per day divided twice daily) or no treatment found significantly less developmental delays via Denver 2 screening examinations at both 6 months and 12 months in the treated versus the nontreated infants [27]. CNS VZV infection may be treated with acyclovir, ganciclovir, or steroids, whereas CNS EBV treatment remains largely supportive. For cases of confirmed HHV-6 encephalitis, ganciclovir or foscarnet may be somewhat efficacious [10••]. Treatments for HHV-7—and HHV-8—associated CNS disease are much less well studied and do not carry specific recommendations [10••]. Outside the Herpesviridae family of CNS diseases, treatment remains largely supportive. Immunomodulatory therapy may play a larger role in the management of pediatric CNS viral infections in the years to come. In the case of congenital CMV infection, vaccine therapy of previously seronegative pregnant females prevented primary maternal infection in 50% of patients compared with placebo in a phase 2 trial using a recombinant CMV glycoprotein B vaccine [28•]. Pass et al. [28•] demonstrated a threefold reduction in congenital CMV among immunized mothers compared with the placebo group, although the number of subjects in this study was relatively small. For prevention of congenital CMV to be truly successful, it must be introduced as part of the routine pediatric vaccination schedule after several years of stringent regulated testing. In cases of CNS influenza or pandemic influenza A (H1N1), oseltamivir may be used (category C). With regard to severe cases of nonpolio enterovirus, intravenous gammaglobulin has shown some efficacy [10••]. Although pediatric CNS viral therapy currently is largely supportive, specific antiviral therapies designed to inhibit viral entry into the CNS (whether vaccine, antibody mediated, or small molecule) will be required to improve neurologic morbidity and mortality.
Emerging Pediatric Neurovirologic Diseases
The importance of emerging viral infections in pediatric neurovirologic disease was highlighted by the recent H1N1 outbreak, which caused significant morbidity and mortality, especially in the young [29, 30]. Among 343 pediatric patients diagnosed with H1N1 in California, presenting features included neurologic symptoms of headache (14%) and altered mental status (4%) [29]. A second case series from Texas in four previously healthy children reported encephalopathy, status epilepticus, and ataxia as presenting features [30]. All four of these patients had normal CSF values, and only one patient showed abnormalities on neuroimaging (cortical T2 hyperintensities on MRI). In addition to H1N1, there are several other neurotropic viruses still emerging, as reviewed by Tyler [31•, 32•] in a recent two-part series. Enterovirus 71, a cause of hand-foot-and-mouth disease in children, may present with neurologic sequelae (opsoclonus–myoclonus, cerebellitis, Guillain-Barré syndrome, transverse myelitis, encephalitis, aseptic meningitis) in up to a third of patients [31•]. JE is of concern because of its high prevalence and robust migration throughout Asia and, more recently, into Australia. JE may present with encephalitis, status epilepticus, or focal limb weakness and has no proven effective treatment, although candidate vaccines are being developed. Two newer neurotropic viruses, Nipah and Hendra, have shown significant neurologic morbidity in affected patients. Nipah and Hendra viruses, members of the Paramyxoviridae family, have been associated with brainstem symptoms and relapsing and/or late-onset encephalitis in up to 8% of survivors [32]. Whether these neurotropic viruses emerge through a change in host, through an increase in geographic range, or by mutational pathogenesis, an epidemiologic knowledge of geographic viral trends remains the best chance of early diagnosis, despite the fact that treatment is largely supportive.
Enlarging Spectrum of Virology-Associated Pediatric CNS Diseases
Acquired Demyelinating Diseases of Childhood
The acquired demyelinating diseases of childhood include a diverse category (optic neuritis, clinical isolated syndrome, acute disseminated encephalomyelitis, multiple sclerosis [MS], neuromyelitis optica, transverse myelitis) with an annual incidence of 0.4 per 100,000 [33]. A neurovirologic etiology has been suggested in many cases, as up to 93% of patients report an infectious prodrome [33]. In the case of pediatric MS, the proposed immune-mediated disease pathophysiology and neuroimaging features that occasionally are similar to those of viral encephalitis have prompted studies to explore a plausible neurovirologic role in MS. The best-studied neurotropic virus in association with both pediatric and adult acquired demyelinating disease is EBV [34–36]. Alotaibi et al. [34] first reported an association in 2004 whereby 83% of pediatric MS patients (n = 30) had remote serologic evidence of EBV infection compared with 42% of controls (n = 143). A follow-up study by Pohl et al. [35] in 2006 in 147 pediatric MS patients revealed evidence of remote infection in 98.6% versus 72.1% of controls (P = 0.001). However, neither pediatric controls nor MS patients showed evidence of acute EBV infection by serology (antibodies directed against EBV early antigen or nuclear antigen). Most recently, Pohl et al. [36] reported intrathecally synthesized EBV antibodies in 26% of childhood-onset MS (N = 43), versus 10% of adult MS (N = 50) and 0% of other pediatric neurologic diseases (N = 32) [36]. Overall, these findings did not support a causative role of EBV infection in pediatric MS in this series because EBV PCR was negative in all samples and 30% to 60% of MS patients showed intrathecally synthesized antibodies to other neurotropic viruses (measles, rubella, VZV). Although none of the data to date have showed conclusive evidence for a specific neurotropic virus in the pathophysiology of pediatric acquired demyelinating disease, it is possible that future research will reveal a common overlap among infection-mediated neuroinflammatory disease mechanisms.
Childhood Epilepsy
Among all neurotropic viruses, HHV-6 is one of the most implicated in the pathophysiology of several childhood epilepsies, including febrile seizures, status epilepticus, and mesial temporal lobe epilepsy [37]. HHV-6 exists as two variants (HHV-6A and HHV-6B) that share greater than 90% sequence homology [38]. HHV-6 is an attractive candidate for childhood-onset epilepsy because more than 90% of children show serologic evidence of primary or latent HHV-6 exposure that occurs at the peak incidence of febrile seizures [38]. HHV-6B, the more commonly associated HHV-6 variant in patients with epilepsy, has been detected in the serum and CSF of pediatric patients with febrile seizures and febrile status epilepticus and is the subject of an extensive review by Theodore et al. [37]. Mesial temporal lobe epilepsy, a common cause of acquired epilepsy, also has been studied with regard to its association with HHV-6B in both pediatric and adult patients. Fotheringham et al. [39•] detected HHV-6B by nested PCR and immunohistochemistry in 8 of 19 pediatric patients with primary mesial temporal lobe epilepsy who underwent surgical resection. Furthermore, primary cultured astrocytes obtained after surgery showed evidence of HHV-6 late antigen by immunofluorescence microscopy, confirming the presence of active viral replication as opposed to latent infection [39•]. One potential mechanism for HHV-6B–mediated epilepsy is the associated downregulation of glutamate transporter EAAT-2 after infection of primary cultured astrocytes with HHV-6 [39•], although there likely are many other possibilities. Although HHV-6 has been shown to be an attractive candidate in the pathophysiology of childhood epilepsy, several other viruses have been detected in association with febrile seizures [37]. Because most patients with epilepsy do not undergo surgery, we must rely on CSF viral detection methods to establish association; ultimately we will need a viable animal model to truly establish a causative link between viral infection and childhood epilepsy.
Primary CNS Malignancies of Childhood
The role of neurotropic viruses in pediatric primary CNS malignancies currently is an area of controversy in neurovirology for several reasons, including an overall lack of understanding of the immunology of CNS tumors, a lack of reproducibility among various researchers regarding viral detection frequency, and the overall implications of viral associations for both diagnosis and treatment of CNS neuro-oncologic disease. Among the many neurotropic viruses, those of the Polyomavirus and Herpesviridae families have been studied most in association with both adult and pediatric primary CNS malignancies. The Polyomavirus family (JC virus, BK virus, SV40 virus), particularly JC virus, is of considerable interest in neuro-oncogenesis, largely based on animal models of JC virus–induced CNS malignancy [40]. JC virus has a seroprevalence of 19% to 50%, increasing with age [41]. In adults, JC virus has been detected in several larger studies by PCR in various tumor subtypes, including oligodendroglioma, glioma, ependymoma, glioblastoma multiforme, and medulloblastoma, in approximately one third to three quarters of cases [40]. JC virus large T antigen, the putative oncogenic protein, has been detected in adult tumors roughly half as often by immunohistochemistry than PCR [40]. The role of JC virus in pediatric CNS tumors has yielded mixed results [42–44]. Krynska et al. [42] detected JC virus DNA and large T antigen in 11 of 23 medulloblastoma cases, whereas Okamoto et al. [43] found viral DNA in 5 of 18 ependymoma and 1 of 5 choroid plexus papilloma cases. On the contrary, Vasishta et al. [44] recently reported that they did not detect JC virus by immunohistochemistry in their series of 22 medulloblastoma patients from India, where seroprevalence is largely unknown. Among the herpesviruses, CMV has generated the most controversy in adult neuro-oncology. It has been detected by PCR, in situ hybridization, and immunohistochemistry with varied frequencies in adult glioblastoma multiforme tumors, which prompted clinical trials using dendritic cell vaccines and antiviral therapies [45]. Much less is known about the frequencies of CMV in a large cohort of pediatric tumors; however, unpublished results from my laboratory suggest CMV DNA is present in a diversity of tumor types and in normal brain by means of nested PCR. More recently, HHV-6 variants were detected in both adult and pediatric primary CNS tumors by both nested PCR and immunohistochemistry, with a predominance of viral antigen detected in glial tumors [46, 47]. However, Neves et al. [48] failed to detect HHV-6 DNA by RT-PCR in a subset of 35 pilocytic astrocytomas. A potential role for HHV-6 in pediatric brain tumors is complicated further by the recent characterization of a chromosomally integrated HHV-6 variant that accounts for a major mode of transmission in congenital HHV-6 infection [49•]. Mechanistically, neurotropic viruses could act via direct cellular transformation or through oncomodulatory mechanisms [40]. Ultimately, multi-institutional studies and reliable animal model systems will be necessary to differentiate viral association from causation in neuro-oncology.
Controversies and Future Directions in Pediatric Neurovirology
Despite an improved appreciation of the vast number of neurotropic viruses and their potential roles in pediatric CNS diseases, there are many controversial areas that likely will be major areas of focus in the next decade. One area of contention is how to reliably distinguish viral causation from association. Part of the difficulty is the inability to differentiate, by current diagnostic modalities, between lytic and latent viral infectivity. Because a large proportion of the population has immunologic evidence of past infection by serology, it is difficult to prove a causative role for acute neurotropic viral infection by macromolecular detection alone. The notion of chronic persistent or latent reactivation of neurotropic viral infection in CNS disease modification may be a more plausible hypothesis. Ultimately, we are limited by the sensitivities of the diagnostic assays in improving our understanding of neurovirologic disease phenotypes. For example, it is presumed that viral macromolecules detected in CSF represent active infection in the CNS parenchyma. However, because the amount of DNA in the CSF often is not quantifiable (eg, in cases of paucicellular CSF), diagnosis depends on the sensitivity of the amplification assay being performed. The detection of intrathecal virus-specific antibodies may have a larger role in diagnostic neurovirology. A more fundamental research question that will need to be addressed is, What is the frequency of neurotropic viruses throughout all ages of pediatric nondiseased brain, and is there a neuroanatomic viral tropism? The answers may help us correlate MRI findings with specific neurovirologic diseases with a goal to develop newer and more sophisticated neuroimaging modalities. A final important aspect of neurovirology research is the design of more specific and efficacious treatment modalities. Current antiviral therapies are given parenterally, with little knowledge of CNS penetration and biodistribution let alone the specific antiviral efficiencies or appropriate duration of therapy. A multitargeted approach involving novel viral replication inhibitors, vaccine-based therapies, and other neuroimmunomodulatory compounds most likely will be the future of neurovirology treatment research.
Conclusions
Pediatric neurovirology is a relatively new subspecialty that spans many disciplines and holds much promise in our understanding of a multitude of pediatric CNS diseases. The development of more sensitive, specific, and comprehensive diagnostic and treatment modalities is necessary for the field of pediatric neurovirology to evolve.
Acknowledgments
Disclosure
No potential conflict of interest relevant to this article was reported.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance, •• Of major importance
- 1.Cannon MJ. Congenital cytomegalovirus (CMV) epidemiology and awareness. J Clin Virol. 2009;46(Suppl 4):S6–S10. doi: 10.1016/j.jcv.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Kimberlin DW. Management of HSV encephalitis in adults and neonates: diagnosis, prognosis and treatment. Herpes. 2007;14:11–16. [PubMed] [Google Scholar]
- 3.Glaser CA, Gilliam S, Schnurr D, et al. In search of encephalitis etiologies: diagnostic challenges in the California Encephalitis Project, 1998–2000. Clin Infect Dis. 2003;36:731–742. doi: 10.1086/367841. [DOI] [PubMed] [Google Scholar]
- 4.Kolski H, Ford-Jones EL, Richardson S, et al. Etiology of acute childhood encephalitis at The Hospital for Sick Children, Toronto, 1994–1995. Clin Infect Dis. 1998;26:398–409. doi: 10.1086/516301. [DOI] [PubMed] [Google Scholar]
- 5.Mailles A, Stahl JP. Infectious encephalitis in France in 2007: a national prospective study. Clin Infect Dis. 2009;49:1838–1847. doi: 10.1086/648419. [DOI] [PubMed] [Google Scholar]
- 6.Yao K, Honarmand S, Espinosa A, et al. Detection of human herpesvirus-6 in cerebrospinal fluid of patients with encephalitis. Ann Neurol. 2009;65:257–267. doi: 10.1002/ana.21611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Theodore WH, Epstein L, Gaillard WD, et al. Human herpes virus 6B: a possible role in epilepsy? Epilepsia. 2008;49:1828–1837. doi: 10.1111/j.1528-1167.2008.01699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kastrup O, Wanke I, Maschke M. Neuroimaging of infections of the central nervous system. Semin Neurol. 2008;28:511–522. doi: 10.1055/s-0028-1083688. [DOI] [PubMed] [Google Scholar]
- 9.Debiasi RL, Tyler KL. Molecular methods for diagnosis of viral encephalitis. Clin Microbiol Rev. 2004;17:903–925. doi: 10.1128/CMR.17.4.903-925.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.•• Tunkel AR, Glaser CA, Bloch KC, et al.: The management of encephalitis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2008, 47:303–327. This article presents an evidence-based review and management recommendations for encephalitis by the Infectious Diseases Society of America. [DOI] [PubMed]
- 11.Lakeman FD, Whitley RJ. Diagnosis of herpes simplex encephalitis: application of polymerase chain reaction to cerebrospinal fluid from brain-biopsied patients and correlation with disease. National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. J Infect Dis. 1995;171:857–863. doi: 10.1093/infdis/171.4.857. [DOI] [PubMed] [Google Scholar]
- 12.Perrott P, Smith G, Ristovski Z, et al. A nested real-time PCR assay has an increased sensitivity suitable for detection of viruses in aerosol studies. J Appl Microbiol. 2009;106:1438–1447. doi: 10.1111/j.1365-2672.2008.04119.x. [DOI] [PubMed] [Google Scholar]
- 13.Notomi T, Okayama H, Masubuchi H, et al. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000;28:E63. doi: 10.1093/nar/28.12.e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parida M, Sannarangaiah S, Dash PK, et al. Loop mediated isothermal amplification (LAMP): a new generation of innovative gene amplification technique; perspectives in clinical diagnosis of infectious diseases. Rev Med Virol. 2008;18:407–421. doi: 10.1002/rmv.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wada K, Mizoguchi S, Ito Y, et al. Multiplex real-time PCR for the simultaneous detection of herpes simplex virus, human herpesvirus 6, and human herpesvirus 7. Microbiol Immunol. 2009;53:22–29. doi: 10.1111/j.1348-0421.2008.00090.x. [DOI] [PubMed] [Google Scholar]
- 16.Shi J, Wu Y, Cai M, Shang S: Rapid diagnosis of herpetic encephalitis in children by PCR microarray technology for simultaneous detection of seven human herpes viruses. Eur J Pediatr 2009 Aug 16 (Epub ahead of print). [DOI] [PubMed]
- 17.Dung NM, Turtle L, Chong WK, et al.: An evaluation of the usefulness of neuroimaging for the diagnosis of Japanese encephalitis. J Neurol 2009 Jul 25 (Epub ahead of print). [DOI] [PubMed]
- 18.Maricich SM, Neul JL, Lotze TE, et al. Neurologic complications associated with influenza A in children during the 2003-2004 influenza season in Houston, Texas. Pediatrics. 2004;114:e626–e633. doi: 10.1542/peds.2004-0143. [DOI] [PubMed] [Google Scholar]
- 19.Baskin HJ, Hedlund G. Neuroimaging of herpesvirus infections in children. Pediatr Radiol. 2007;37:949–963. doi: 10.1007/s00247-007-0506-1. [DOI] [PubMed] [Google Scholar]
- 20.Crawford JR, Chang T, Lavenstein BL, Mariani B. Acute and chronic magnetic resonance imaging of human herpesvirus-6 associated encephalitis. J Pediatr Neurol. 2009;7:367–373. [Google Scholar]
- 21.Davis LE, DeBiasi R, Goade DE, et al. West Nile virus neuroinvasive disease. Ann Neurol. 2006;60:286–300. doi: 10.1002/ana.20959. [DOI] [PubMed] [Google Scholar]
- 22.Sawlani V. Diffusion-weighted imaging and apparent diffusion coefficient evaluation of herpes simplex encephalitis and Japanese encephalitis. J Neurol Sci. 2009;287:221–226. doi: 10.1016/j.jns.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 23.Yoshinari S, Hamano S, Ito T, Eto Y. MRI-diffusion weighted images of encephalopathy associated with human herpesvirus 6 infection [in Japanese] No To Hattatsu. 2005;37:374–379. [PubMed] [Google Scholar]
- 24.Provenzale JM. vanLandingham KE, Lewis DV, et al.: Extrahippocampal involvement in human herpesvirus 6 encephalitis depicted at MR imaging. Radiology. 2008;249:955–963. doi: 10.1148/radiol.2492071917. [DOI] [PubMed] [Google Scholar]
- 25.James SH, Kimberlin DW, Whitley RJ. Antiviral therapy for herpesvirus central nervous system infections: neonatal herpes simplex virus infection, herpes simplex encephalitis, and congenital cytomegalovirus infection. Antiviral Res. 2009;83:207–213. doi: 10.1016/j.antiviral.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elbers JM, Bitnun A, Richardson SE, et al. A 12-year prospective study of childhood herpes simplex encephalitis: is there a broader spectrum of disease? Pediatrics. 2007;119:e399–e407. doi: 10.1542/peds.2006-1494. [DOI] [PubMed] [Google Scholar]
- 27.Oliver SE, Cloud GA, Sanchez PJ, et al. Neurodevelopmental outcomes following ganciclovir therapy in symptomatic congenital cytomegalovirus infections involving the central nervous system. J Clin Virol. 2009;46(Suppl 4):S22–S26. doi: 10.1016/j.jcv.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.• Pass RF, Zhang C, Evans A, et al.: Vaccine prevention of maternal cytomegalovirus infection. N Engl J Med 2009, 360:1191–1199. This phase 2 randomized double-blind control trial of a recombinant CMV vaccine demonstrated 50% efficacy at 42 months among CMV-seronegative females within 1 year after giving birth. [DOI] [PMC free article] [PubMed]
- 29.Louie JK, Acosta M, Winter K, et al. Factors associated with death or hospitalization due to pandemic 2009 influenza A (H1N1) infection in California. JAMA. 2009;302:1896–1902. doi: 10.1001/jama.2009.1583. [DOI] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention Neurologic complications associated with novel influenza A (H1N1) virus infection in children—Dallas, Texas, May 2009. MMWR Morb Mortal Wkly Rep. 2009;58(28):773–778. [PubMed] [Google Scholar]
- 31.• Tyler KL: Emerging viral infections of the central nervous system: part 1. Arch Neurol 2009, 66:939–948. This is part 1 of an excellent review on emerging infections of the CNS. [DOI] [PMC free article] [PubMed]
- 32.• Tyler KL: Emerging viral infections of the central nervous system: part 2. Arch Neurol 2009, 66:1065–1074. This is part 2 of an excellent review on emerging infections of the CNS. [DOI] [PMC free article] [PubMed]
- 33.Tenembaum S, Chitnis T, Ness J. Hahn JS: Acute disseminated encephalomyelitis. Neurology. 2007;68:S23–S36. doi: 10.1212/01.wnl.0000259404.51352.7f. [DOI] [PubMed] [Google Scholar]
- 34.Alotaibi S, Kennedy J, Tellier R, et al. Epstein-Barr virus in pediatric multiple sclerosis. JAMA. 2004;291:1875–1879. doi: 10.1001/jama.291.15.1875. [DOI] [PubMed] [Google Scholar]
- 35.Pohl D, Krone B, Rostasy K, et al. High seroprevalence of Epstein-Barr virus in children with multiple sclerosis. Neurology. 2006;67:2063–2065. doi: 10.1212/01.wnl.0000247665.94088.8d. [DOI] [PubMed] [Google Scholar]
- 36.Pohl D, Rostasy K, Jacobi C, et al.: Intrathecal antibody production against Epstein-Barr and other neurotropic viruses in pediatric and adult onset multiple sclerosis. J Neurol 2009 Aug 28 (Epub ahead of print). [DOI] [PMC free article] [PubMed]
- 37.Theodore WH, Epstein L, Gaillard WD, et al. Human herpes virus 6B: a possible role in epilepsy? Epilepsia. 2008;49:1828–1837. doi: 10.1111/j.1528-1167.2008.01699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caserta MT, Mock DJ, Dewhurst S. Human herpesvirus 6. Clin Infect Dis. 2001;33:829–833. doi: 10.1086/322691. [DOI] [PubMed] [Google Scholar]
- 39.• Fotheringham J, Donati D, Akhyani N, et al.: Association of human herpesvirus-6B with mesial temporal lobe epilepsy. PLoS Med 2007, 4:e180. This article presents supporting evidence for the role of HHV-6 in epilepsy. HHV-6B was demonstrated by PCR in 11 of 16 patients with mesial temporal lobe epilepsy, and viral antigen was detected in primary astrocytes ex vivo in culture. [DOI] [PMC free article] [PubMed]
- 40.White MK, Gordon J, Reiss K, et al. Human polyomaviruses and brain tumors. Brain Res Rev. 2005;50:69–85. doi: 10.1016/j.brainresrev.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 41.Knowles WA, Pipkin P, Andrews N, et al. Population based study of antibody to the human polyomaviruses BKV and JCV and the simian polyomavirus SV40. J Med Virol. 2003;71:115–123. doi: 10.1002/jmv.10450. [DOI] [PubMed] [Google Scholar]
- 42.Krynska B, Del Valle L, Croul S, et al. Detection of human neurotropic JC virus DNA sequence and expression of the viral oncogenic protein in pediatric medulloblastomas. Proc Natl Acad Sci U S A. 1999;96:11519–11524. doi: 10.1073/pnas.96.20.11519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Okamoto H, Mineta T, Ueda S, et al. Detection of JC virus DNA sequences in brain tumors in pediatric patients. J Neurosurg. 2005;102:294–298. doi: 10.3171/ped.2005.102.3.0294. [DOI] [PubMed] [Google Scholar]
- 44.Vasishta RK, Pasricha N, Nath A, Sehgal S. The absence of JC virus antigens in Indian children with medulloblastomas. Indian J Pathol Microbiol. 2009;52:42–45. doi: 10.4103/0377-4929.44961. [DOI] [PubMed] [Google Scholar]
- 45.Miller G. Brain cancer. A viral link to glioblastoma? Science. 2009;323:30–31. doi: 10.1126/science.323.5910.30. [DOI] [PubMed] [Google Scholar]
- 46.Crawford JR, Santi MR, Thorarinsdottir HK, et al. Detection of human herpesvirus-6 variants in pediatric brain tumors: association of viral antigen in low grade gliomas. J Clin Virol. 2009;46:37–42. doi: 10.1016/j.jcv.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crawford JR, Santi MR, Cornelison R, et al. Detection of human herpesvirus-6 in adult central nervous system tumors: predominance of early and late viral antigens in glial tumors. J Neurooncol. 2009;95:49–60. doi: 10.1007/s11060-009-9908-2. [DOI] [PubMed] [Google Scholar]
- 48.Neves AM, Thompson G, Carvalheira J, et al. Detection and quantitative analysis of human herpesvirus in pilocytic astrocytoma. Brain Res. 2008;1221:108–114. doi: 10.1016/j.brainres.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 49.• Hall CB, Caserta MT, Schnabel K, et al.: Chromosomal integration of human herpesvirus 6 is the major mode of congenital human herpesvirus 6 infection. Pediatrics 2008,122:513–520. The authors demonstrate that chromosomally integrated HHV-6 is the major cause of congenital HHV-6 infection, occurring in 1% of all newborns. [DOI] [PubMed]


