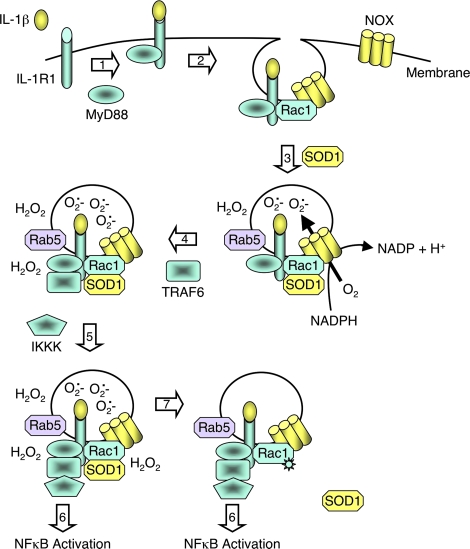
FIG. 2.
IL-1β mediates NFκB activation through redoxosomal activation. Schematically drawn are steps involved in the redox-activation of NFκB by IL-1β (82, 97, 100). Step 1: IL-1β binding to the IL-1 receptor (IL-1R1) facilitates recruitment of the effector MyD88 to the plasma membrane. Step 2: MyD88 binding to the ligand/receptor complex facilitates dynamin-dependent endocytosis and recruitment of Rac1 to the receptor. Rac1 is responsible for co-endocytosis of the Nox complex with the ligand-activated receptor. Step 3: Endocytosis of the IL-1R1/Nox complex leads to NADPH-dependent O2•− production in the endosomal lumen. In the case of Rab5 recruitment, is currently unclear whether it occurs at the endosome or the plasma membrane. However, superoxide production initiates in the Rab5-positive, early endosomal compartment. Additionally, SOD1 is recruited to the cytoplasmic face of the redoxosome, where it binds to Rac1 and stabilizes the GTP-bound active form of Rac1. Step 4: The localized production of ROS by newly formed redoxosomes facilitates H2O2-mediated recruitment of TRAF6 to IL-1R1 on the redoxosome surface. Extra-redoxosomal H2O2 may be generated either in the cytoplasm, following exit of O2•− from the lumen via chloride channels, or within the redoxosome lumen by spontaneous dismutation of O2•− within the lumen, followed by diffusion across the redoxosomal membrane. Step 5: The IL-1R1/MyD88/TRAF6 complex is now competent to recruit IKK kinases (IKKKs), and the recruited IKKKs can then phosphorylate cytoplasmically localized IKK complexes to activate NFκB. Step 6: As ROS levels outside the redoxosome rise, Rac1 is oxidized and SOD1 disengages. The uncoupling of SOD1 from Rac1 leads to: enhanced GTP hydrolysis by Rac1, inactivation of the Nox complex, and the termination of ROS production. This redox-dependent uncoupling of SOD1 from Rac1 appears to be defective in certain ALS-associated SOD1 mutants, and may lead to increases redox stress in the context of stimulating ligands that utilize redoxosomal pathways. Similar redoxosomal mechanisms appear to control TNFα-mediated activation of NFκB (83, 97). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).