Abstract
Nocturnal hawkmoths are known for impressive visually guided behaviours in dim light, such as hovering while feeding from nectar-bearing flowers. This requires tight visual feedback to estimate and counter relative motion. Discrimination of low velocities, as required for stable hovering flight, is fundamentally limited by spatial resolution, yet in the evolution of eyes for nocturnal vision, maintenance of high spatial acuity compromises absolute sensitivity. To investigate these trade-offs, we compared responses of wide-field motion-sensitive neurons in three species of hawkmoth: Manduca sexta (a crepuscular hoverer), Deilephila elpenor (a fully nocturnal hoverer) and Acherontia atropos (a fully nocturnal hawkmoth that does not hover as it feeds uniquely from honey in bees' nests). We show that despite smaller eyes, the motion pathway of D. elpenor is tuned to higher spatial frequencies and lower temporal frequencies than A. atropos, consistent with D. elpenor's need to detect low velocities for hovering. Acherontia atropos, however, presumably evolved low-light sensitivity without sacrificing temporal acuity. Manduca sexta, active at higher light levels, is tuned to the highest spatial frequencies of the three and temporal frequencies comparable with A. atropos. This yields similar tuning to low velocities as in D. elpenor, but with the advantage of shorter neural delays in processing motion.
Keywords: night vision, hawkmoth, hovering, intracellular electrophysiology
1. Introduction
Hovering is a challenging behaviour not only because it requires high energy and precise control of aerodynamical forces (Ellington 1984a,b; Willmott & Ellington 1997), but also because it places strict demands on the visual system. To siphon nectar effectively, a hovering hawkmoth must maintain a minimal distance to its flower. If it drifts in the air, it must counter that motion before it is carried out of range—roughly the length of its proboscis (Farina et al. 1994; Sprayberry & Daniel 2006). Visually estimating potentially tiny deviations from a motionless hovering state requires sensitivity to low-velocity visual motion (O'Carroll et al. 1996, 1997).
Many hawkmoths are adept at hovering and obtaining nectar even on moonless nights (Pittaway 1993). Performing any visual task in the dark is problematic (Warrant 2008), hovering particularly so. This is because good spatial acuity is required for stabilizing the images of flowers during hovering, and in dim light this depends critically on the eyes having adequate sensitivity to light. However, a tenet of eye design is that sensitivity trades with spatial acuity (Land 1981; Warrant & McIntyre 1992; Land & Nilsson 2002): as light levels fall, the optimal spatial acuity of an eye—the acuity that maximizes the number of discernible scenes—also falls (Snyder et al. 1977a,b). However, adequate spatial resolution is integral to detecting the low velocities of hovering. The velocity of a drifting sinusoidal grating is given by the quotient of its temporal and spatial frequencies, V = ft/fs, and any natural image can be considered to be the sum of many gratings. So detecting low velocities depends on some sensitivity to either high spatial or low temporal frequencies. For a hovering nocturnal insect, already handicapped by the inherently poor spatial resolution of compound eyes (Kirschfeld 1976), further losses in spatial acuity required for night vision may sacrifice its ability to steady itself with visual cues. The alternative strategy, detecting low velocities with an enhanced sensitivity to very low temporal frequencies, might slow the quick responses necessary to hold station in front of a wind-tossed flower.
To investigate the trade-offs imposed on motion detection by the evolution of hovering and nocturnal vision, this paper compares wide-field motion-detecting neurons in three hawkmoths: the nocturnal hoverer Deilephila elpenor, the crepuscular hoverer Manduca sexta and the non-hovering nocturnal cleptoparasite Acherontia atropos. Manduca sexta is a large moth with large eyes (eye diameter approx. 4 mm), known to feed at dusk and dawn by hovering steadily while feeding from flowers. Deilephila elpenor feeds similarly, but has a much smaller body and correspondingly smaller eyes (eye diameter approx. 2.5 mm), and is a truly nocturnal moth noted for spectacular dim-light visual abilities (Pittaway 1993; Kelber et al. 2002). Acherontia atropos, a large moth like M. sexta (eye diameter approx. 5 mm), is fully nocturnal and a long-distance migrator; however, it does not hover in front of flowers. Instead, it feeds by entering honeybee nests and parasitizing the food cells (Kitching & Cadiou 2000; Kitching 2003).
2. Material and methods
(a). Moths
Manduca sexta were reared from the colony at the University of Washington, Seattle. Acherontia atropos were kindly provided as first instar larvae, and D. elpenor as pupae, by Dr Almut Kelber of Lund University in Sweden. We placed the pupae on a 12 : 12 light–dark cycle. We used moths 2–4 days post-emergence, only after confirming that all parts were normally formed and clean, and that each could fly normally.
(b). Anatomy
In order to compare physiological differences between species, it was necessary to take into account any anatomical differences also present. To do this we measured body length, eye diameter, facet diameter and interommatidial angle from specimens obtained from the collection of the Lund University Zoological Museum. We used a Zeiss stereomicroscope (Stemi SV 6 with a Plan S 1 × objective), with attached Sony digital camera (Cyber-shot 5 MP, model DSC-F707). From the digital photos, we then measured eye diameter and facet diameter using the measurement tool in Photoshop (Adobe Systems, Inc., USA). We calculated interommatidial angles using the relation Δφ (in radians) = (facet diameter/eye radius), where eye radius is equal to half the eye diameter, assuming a hemispherical eye (a valid assumption for sphingid moths). The facets chosen to measure facet diameter were in the central lateral eye.
(c). Electrophysiology
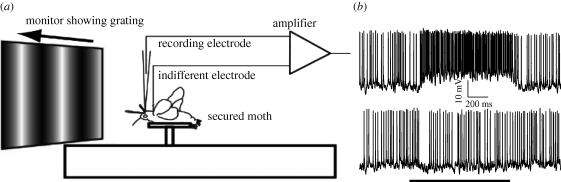
Moths were immobilized with plastic tubes fitted around their bodies and melted wax applied to stabilize their head positions. We made a hole behind the left antenna and removed small amounts of connective tissue to expose the surface of the brain above the optic lobe. Neurons were impaled intracellularly by advancing quartz electrodes, pulled with a Sutter P-2000 and filled with 1 M KCl, onto the proximal area of the optic lobe, the region of the third optic ganglion (the lobula). Electrode resistance was initially around 80 MΩ, but decreased to 5–20 MΩ after penetrating tracheal tubes and surface membranes, and presumably breaking slightly at the tip. Acherontia atropos has a brain pushed farther back in the head, probably because room in the head is allotted to muscular sucking structures. Aside from this difference, the tracheal patterns on the brain in the posterior head provide reliable landmarks to locate motion-sensitive neurons. The moths were placed so the right eye viewed the stimulus at roughly 45° from its midline (figure 1a).
Figure 1.
Experimental apparatus and sample data traces. (a) Each moth was secured approximately 10 cm in front of a 10 cm square monitor. The monitor emitted light of intensity 40 cd m−2, updated at 183 Hz, and displayed drifting sinusoidal gratings of specified orientation, spatial frequency and temporal frequency. The arrow indicates the direction of drift. A quartz intracellular electrode was inserted behind the antennae recorded from motion-sensitive neurons in the third optic ganglion (the lobula) of the ipsilateral eye. (b) Sample traces from M. sexta show the baseline spike rate before stimulation while moths viewed a blank screen at mean luminance, and the response while the sinusoid moved in the preferred (upper trace) or anti-preferred (lower trace) direction. The scale bar below the upper trace denotes 200 ms and 10 mV, and the scale bar below the lower trace denotes duration of the moving stimulus (2 s).
(d). Stimulus
We generated moving sinusoidal gratings on a 10 × 10 cm Textronix 608 oscilloscope monitor (figure 1a). When placed about 10 cm in front of the moth's right eye, it covered a square visual patch subtending 53° on each side. The gratings have the advantage that their spatial and temporal frequencies are entirely separable, such that we can alter either variable without changing the other. Further, even complex visual scenes can be decomposed into a linear sum of moving sinusoidal gratings (they form a basis of time-varying visual scenes), with a scalar multiple determining the maximal intensity, and therefore the contrast, of each. The monitor updated at 183 Hz produced a brightness of 40 candelas per square metre (cd m−2) and was controlled by an InnisFree Picasso stimulus generator, which allowed precise specification of orientation, phase and spatial frequency. Each moving grating appeared for 2 s at a contrast of 0.5 and was preceded by 3 s of blank screen at mean luminance. When a neuron responded to the gratings, we displayed them in 24 different orientations, in random order, to characterize the optimal drift direction, and used this direction for all subsequent experiments. We then varied the spatial frequency between 0.01 and 1 cycles deg−1, and temporal frequency between 0.1 and 50 Hz, both ranges divided into 20 logarithmically spaced intervals. Our earlier work has verified that the velocity tuning of insect neurons to spectrally broadband stimuli is predicted well by their tuning to the spatial and temporal frequency of grating patterns (Dror et al. 2001), so the measured differences in velocity optima are likely to reflect differences in velocity tuning for natural scenes. One caveat, however, is that the optima for scenes with 1/f statistics, such as natural images, while still predictable, will probably be two to three times higher than those for narrow-band sinusoids, as we have observed for the hoverfly (Straw et al. 2008).
(e). Response analysis
Prior to each stimulus, we displayed a screen at the mean luminance for 3 s and measured baseline spike rate for the final 1 s. During stimulation, we measured spike rate through the entire 2 s stimulus, but discarded the first and last 250 ms to minimize problems with onset artefacts and motion adaptation. We then built spatial and temporal frequency response curves, sampling at each combination six times. These techniques produced spatial and temporal frequency tuning curves but required stable recordings of 30 min or longer. In the optimal case, when all (400) possible combinations of temporal and spatial frequencies were tested to produce a three-dimensional response surface, recordings required 2 h to complete.
3. Results
Measurements of museum specimens (table 1) confirm that, by body length, M. sexta and A. atropos are considerably larger moths than D. elpenor, and this manifests in their eye sizes. The diameter of A. atropos's eye is nearly twice that of D. elpenor's, with M. sexta falling in-between at the upper end of the range. In compound eye optics, bigger eyes have additional room to hold either larger facets, or more facets with smaller interommatidial angles. Larger facets, which collect more light, are usually associated with sensitivity (although sensitivity in a superposition eye is ultimately determined by the area of the superposition aperture), while greater numbers of facets, which sample the visual scene more densely, are associated with acuity. These moths appeared to follow both strategies according to their size: A. atropos, with the largest eyes, has both the largest facet diameter (36 µm) and the smallest interommatidial angles; M. sexta has a comparable but smaller facet diameter and comparable but larger interommatidial angles; and D. elpenor, with the smallest eyes, has both the smallest facet size and largest interommatidial angles.
Table 1.
Anatomical data from three species of sphingid moths: A. atropos (n = 4), D. elpenor (n = 5) and M. sexta (n = 4). Values reported are mean ± error, where the error is the greatest deviation between the mean and any original data point.
| moth | body length (mm) | eye diameter (mm) | facet diameter (µm) | Δφ (degrees) |
|---|---|---|---|---|
| A. atropos | 54 ± 5 | 4.9 ± 0.3 | 36 ± 2 | 0.87 ± 0.07 |
| D. elpenor | 31 ± 2 | 2.6 ± 0.1 | 29 ± 1 | 1.31 ± 0.08 |
| M. sexta | 43 ± 7 | 4.1 ± 0.4 | 34 ± 3 | 0.96 ± 0.04 |
During intracellular recording, a typical baseline spike rate in lobula neurons of all species was 10–40 spikes s−1, and an optimal motion stimulus at high contrast can increase this rate by a factor of 2–10 (figure 1b). While baseline varied between cells, it remained relatively constant within a cell for the duration of experiments. Baseline firing rates rarely varied by more than 10 spikes s−1, so we pooled normalized data from multiple recordings after subtracting spontaneous activity.
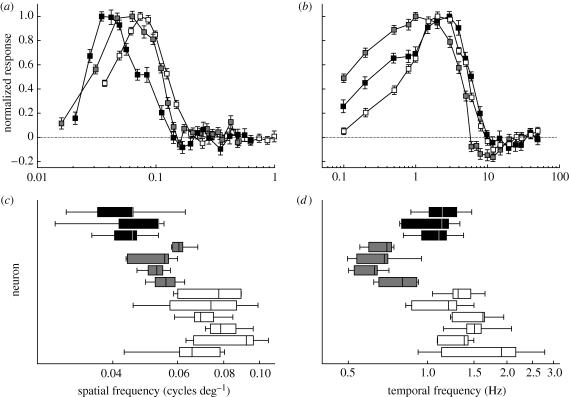
We obtained spatial and temporal tuning curves from four D. elpenor neurons, two from each of two moths, six M. sexta neurons from five different moths and three A. atropos neurons from three different moths. Figure 2a shows the mean spatial tuning curves for each of the three moth species. Manduca sexta, the species regularly active at the highest light levels, is most responsive at the highest spatial frequencies, peaking at 0.074 cycles deg−1. The fully nocturnal A. atropos, likewise, has peak response to the lowest spatial frequencies, at 0.035 cycles deg−1. Deilephila elpenor, however, which has smaller eyes and is active under light at least as dim as A. atropos, has peak spatial tuning higher than A. atropos, at 0.063 cycles deg−1. Moths did not respond to spatial frequencies higher than approximately 0.2 cycles deg−1. Figure 2b shows the mean temporal frequency tuning curves for each moth, which exhibit different patterns. Acherontia atropos has a peak response at the highest temporal frequencies, around 3 Hz. Manduca sexta has a lower peak response (approx. 2 Hz) with an otherwise similarly shaped tuning to A. atropos. Deilephila elpenor has a peak at the lowest frequencies (1 Hz) and a notably broader response extending sensitivity to lower temporal frequencies, with 50 per cent maximal responses at the lowest temporal frequency tested (0.1 Hz). Temporal frequencies in all species drop off quickly above the peak, and no positive spiking responses were detected above 10 Hz.
Figure 2.
Response tuning of wide-field motion-detecting neurons in each moth. Upper panels show the normalized responses of neurons to sinusoidal gratings at different spatial (a) and temporal (b) frequencies, with error bars showing the standard error of the mean. The normalization mapped the maximal mean response to 1, and the baseline response to 0. Temporal frequencies were tested at identical levels, but spatial frequencies varied slightly because the exact monitor distance changed somewhat between experiments. The non-hovering A. atropos was tuned to the lowest spatial frequencies, while crepuscular M. sexta was tuned to the highest. However, nocturnal hovering D. elpenor was tuned to lower temporal frequencies than the other moths. Lower panels show the ‘centre of mass’ spatial (c) and temporal (d) frequency of each neuron tested, calculated by averaging each frequency weighted by its relative response. Because centre of mass calculations weight the whole curve, their centres are not necessarily identical to the peaks of curves in the panels above (a, b). Each box shows the median and first and third quartiles, and whiskers show the range of the six trials for each neuron. Black: A. atropos; grey: D. elpenor; white: M. sexta.
Peak response is only one of many measurements in the tuning curve, and often the trial-to-trial peak varied within a single neuron. In order to determine if the variation in peak responses represents real differences in cell tuning, we further examined the cells by comparing weighted centres. In this case, we took the mean frequency, weighted by response, for the six trials of each cell. The advantage of this method is that all the measurements contribute to the comparison, rather than just the peak. Figure 2c,d shows a series of box plots with this result. The median of the six trials is shown by a dividing line, a box outlines the first and third quartiles, and whisker bars span the range. Figure 2c shows the spatial tuning and, while there is some overlap between ranges of different species, the medians of each cell confirm the sequence in figure 2a: M. sexta is sensitive to the highest spatial frequencies of the three, A. atropos to the lowest and D. elpenor to the frequencies in-between. Figure 2d shows the weighted average temporal frequencies. Once again there is overlap in the ranges and even the quartiles, but the medians of each cell are strictly ordered by species. However, in this case the sequence is at odds with the peak values from figure 2b. Deilephila elpenor is still responsive to the lowest temporal frequencies of the three, but because of the shape of the response curve, M. sexta by this measure is responsive to the highest temporal frequencies and A. atropos to the frequencies in-between.
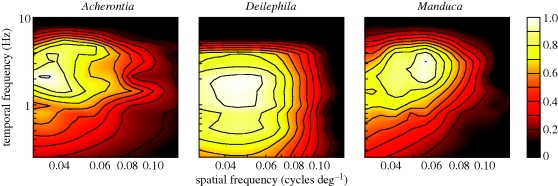
On occasion, when recordings lasted much longer, we could collect data for an entire spatio-temporal response surface. Figure 3 shows the response surfaces for each moth, illustrating the change in spike rate as we sampled among multiple combinations of spatial and temporal frequencies. Although these surfaces are based on fewer cells (three for M. sexta, two for A. atropos and one for D. elpenor), they offer a more complete illustration of wide-field tuning properties. The results again show that A. atropos motion detection is tuned to lower spatial frequencies than the other moths. Manduca sexta is responsive in a similar range of temporal frequencies as A. atropos, but its spatial frequency response is shifted to the finest gratings of the three. Finally, D. elpenor motion detection peaks at middle spatial frequencies between the other two moths, but has much broader tuning in the temporal domain and thus responds to noticeably lower temporal frequencies.
Figure 3.
Spatio-temporal response surfaces and contours for each moth. Responses were measured as in the previous figure, but the moving gratings were systematically varied with combinations of spatial and temporal frequencies, presented in a random order. Deilephila elpenor again shows a peak temporal response below the other moths, while A. atropos and M. sexta are comparable. Acherontia atropos and M. sexta, however, have widely different peak spatial responses, A. atropos the lowest of the three and M. sexta the highest. Colour scale represents normalized spike rate.
4. Discussion
For an ideal eye viewing an image at a given light level and motion speed, there is an optimal spatial acuity that will maximize the information flow (Snyder et al. 1977a,b). However, real animals experience wide ranges of light level and motion speed, so the eventual structure of the visual system is a matter of trade-offs imposed on their evolution. A nocturnal insect benefits from an environment with potentially fewer predators and competitors (Wcislo et al. 2004), and a hovering insect benefits from nectar searches that are potentially quicker and more effective (Dreisig 1997). But nocturnal vision and hovering make contrary demands on some parameters of the visual system. Our aim was to investigate the compromises this brings about in the motion pathway by examining three similar moths: one with nocturnal vision, one with steady hovering and one with both.
Because we were interested in studying the inherent tuning of the filters involved in motion detection, rather than the limits of vision per se, we studied all three species in a light-adapted state and at a moderate luminance (40 cd m−2). Since individual photoreceptors in hawkmoth superposition eyes collect light from hundreds of facets (e.g. Warrant et al. 1999; Kelber et al. 2002), if one moth possessed even a partly open pupil, it would deliver substantially more light to the retina, despite identical brightness at the surface of the eye. However, since the pupil of the superposition eye in these moths is fully closed at this luminance, the differences in spatial tuning that we observed are not easily explained by optical differences in spatial resolution in the superposition mechanism, as observed previously in nocturnal and crepuscular dung beetles (McIntyre & Caveney 1998). Instead, we suggest these reflect differences in the underlying hard wiring for spatial interactions on scales that span numerous ommatidial axes.
(a). The demands of a nocturnal hovering strategy
Both D. elpenor and A. atropos are active in much dimmer light than crepuscular M. sexta, and this would tend to favour lower spatial and temporal acuity (Van Hateren 1993; Warrant 1999), just as our own rod-dominated night vision, although highly sensitive, is poorly resolved and sluggish. Consistent with this result, D. elpenor and A. atropos show peak and median responses to lower spatial frequencies than M. sexta (figure 2a,c) in their motion pathways. Deilephila elpenor, a smaller moth with smaller eyes and greater interommatidial angles than A. atropos, might be expected to respond to lower spatial frequencies, but this is not the case: D. elpenor has a significant response at higher spatial frequencies (figure 2a,c). The median responses of both D. elpenor and A. atropos are located at lower temporal frequencies than those found in M. sexta, but D. elpenor's responses are consistently lower than those of A. atropos. Further, if one only examines the peak responses, A. atropos is placed in a similar temporal frequency range as M. sexta. Acherontia atropos even has a slightly higher response peak, despite its activity in far dimmer light.
These observations match the predictions of eye scaling and nocturnal vision, which suggest that M. sexta, with more light available and a larger eye, would be tuned to analyse higher spatial and temporal frequencies than the diminutive, nocturnal D. elpenor. Given its larger body and eye size, and smaller interommatidial angles, A. atropos could potentially exploit greater spatial acuity than D. elpenor at the same light levels; yet in the motion pathway we observe the opposite, with A. atropos having the lowest spatial optimum. The fact that M. sexta and D. elpenor can hold still in the air well enough to feed, and A. atropos cannot, helps make sense of this discrepancy. Deilephila elpenor, selected for hovering at night, is unable to sacrifice as much spatial acuity as A. atropos, selected for fast flight at night, without compromising low velocity sensitivity. That leaves only one option for D. elpenor: a low temporal frequency optimum, which aids both vision in dim light and sensitivity to low velocities. As a result, D. elpenor neurons responded maximally to gratings moving around 15 deg s−1, in contrast to 85 deg s−1 for A. atropos. The downside for D. elpenor is that the longer time constant in the temporal domain means a more sluggish response and hence a slower control loop for the optomotor reflex. Manduca sexta, with the luxury of being able to operate at somewhat higher luminance and possessing a bigger overall eye, achieves a higher spatial optimum, thus permitting responses to similarly low velocities, but with less sluggish temporal acuity. Indeed, it is interesting to note that the velocity optimum in M. sexta is similar to those published for two diurnal sphingid taxa, Hemaris and Macroglossum (O'Carroll et al. 1996, 1997), yielding optimal responses to similarly low velocities (27 deg s−1 for M. sexta and approx. 20 deg s−1 for Hemaris and Macroglossum).
(b). Where does the acuity difference originate?
Of the enormous range of spatial and temporal frequencies of natural light falling onto the eye, only a comparatively narrow band is encoded by the retina. Whereas optimal spatio-temporal filtering for vision in dim light is an integral component of early visual processing (Van Hateren 1993), optimal filtering for hovering is specific to the motion-detection pathway. Because the conflicting demands of nocturnal vision and hovering vision are restricted to the motion pathway, the frequency tuning observed in motion-sensitive neurons may or may not be manifested upstream. Ultimately, the passage of information through the nervous system costs energy (Laughlin 2001), and if the wide-field motion pathway were the only channel of visual processing, there would be no reason to transduce spatial and temporal frequencies outside the range processed by the motion-detecting neurons. However, parallel pathways in the moth visual system perform other tasks, such as detecting looming stimuli (Farina et al. 1994; Wicklein & Strausfeld 2000; Sprayberry & Daniel 2006) or small targets (Collett 1971), and these might be optimized for visual frequencies outside the bands used by the wide-field motion pathway. Information not collected by the eye, or lost during early visual processing, cannot be recovered downstream. Therefore, these moths might be expected to collect broadband spatial and temporal information at early levels of visual processing and then filter it differently in different visual pathways.
If this is the case, then filtering probably occurs after the level of the retina. Even though the extent of this filtering, relative to that performed by the retina, is currently difficult to quantify due to our lack of knowledge of the optical properties and physiology of photoreceptors in nocturnal moths, we can nevertheless make some preliminary observations. First, in the temporal domain, D. elpenor's motion-detecting neurons did not respond to temporal frequencies above approximately 5 Hz. Even though the photoreceptors of nocturnal and/or slow-flying insects are typically tuned to lower temporal frequencies to improve visual reliability, they usually respond vigorously to considerably higher temporal frequencies than 5 Hz (e.g. nocturnal crane flies, Laughlin & Weckstrom 1993; cockroaches, Heimonen et al. 2006; nocturnal bees, Frederiksen et al. 2008), and this is also very likely to be the case for the photoreceptors of D. elpenor. These higher frequencies would then be available for other visual channels with different needs. Likewise, A. atropos neurons did not respond to spatial frequencies above about 0.1 cycles deg−1. Yet this coarse acuity is not the result of A. atropos having vestigial eyes—it is a migratory moth with large eyes; charged with the difficult task of identifying and entering bees' nests at night, and it could conceivably benefit from having higher spatial acuity. Its large eyes allow smaller interommatidial angles (approx. 0.9°) than are found in the similarly nocturnal D. elpenor (approx. 1.3°), but D. elpenor's motion-detecting neurons nonetheless responded to higher spatial frequencies. The lower spatial frequency response range found in A. atropos may arise due to low-pass spatial filtering at an earlier level of visual processing; for example, via the lateral connections of amacrine cells in the lamina, or by sub-sampling of the elementary motion detectors (see Pick & Buchner 1979). However, these arguments must be qualified by the fact that our experiments were performed in relatively light-adapted conditions (40 cd m−2), and that the nature and extent of spatio-temporal filtering in the retina and the lobula is likely to become more pronounced as light levels fall. A thorough investigation of visual performance in nocturnal hawkmoths at dimmer and more behaviourally relevant light intensities is thus highly warranted.
(c). Other sensory inputs assist in controlling flight
Vision is only one of several senses known to affect flight in insects. Dipterans, for example, rely on gyroscopic measurements from the halteres to determine self-rotation (Pringle 1948), and moths are now known to obtain similar measurements from their antennal motions (Sane et al. 2007). Blowflies are also known to infer rotational motion from another visual pathway that derived from the low-resolution ocelli located between their compound eyes (Parsons et al. 2006). Flies also use wind to infer their own self-motion and wind modifies their responses to visual cues (Budick et al. 2007). Mechanosensory cues are also important for moths when they make contact with a flower (Goyret & Raguso 2006). Moths and many other insects can follow odour plumes of quite low concentration over surprising distances (Murlis et al. 1992). Odour additionally alters the responses to visual cues in flies (Chow & Frye 2008). We can expect that these and similar pathways are of heightened importance in species active in dim light.
However, vision is an important sense for controlling flight even in many nocturnal animals. For example, bees that forage at night will not do so without adequate light (Kelber et al. 2006). With apposition eyes, bees see much more poorly in dim light than moths (Land 1981; Land & Nilsson 2002; Warrant 2004), yet the nocturnal sweat bee Megalopta uses only visual cues when approaching its nest, even in severely dim conditions (Warrant et al. 2004). These factors are even more critical for hovering animals, since they must maintain near-zero translational velocity (although substantial rotational velocity might be tolerated; see Kern & Varjú 1998). Odour plumes, which can be reliable trails to distant resources, are structurally complex and quickly varying at close ranges (Murlis et al. 1992), and are thus unhelpful for holding a static position. At very low speeds, wind signals from self-motion disappear. Acceleration detection can produce a fast response to unplanned perturbations, but inferring translational velocity from this requires mathematical integration. For a small animal pushed by tiny forces, this signal is transient and may be noisy or below threshold; thus integration might amplify this error into a highly unreliable estimate of velocity. This problem is familiar to that faced by ‘blue water divers’, who, lacking visual cues, can be carried substantial distances by currents they were unaware of (Haddock & Heine 2005). Assuming the visual environment is largely stable, optic flow directly estimates self-motion, and although this carries some estimation error, it does not impart the accumulating error inherent in integration. Rotational motion offers a persistent force signal to a constant motion, but also provides a much stronger visual signal, since objects at arbitrary distances, such as the moon, appear to move during rotational self-motion. Although in some insects, such as dragonflies, ocelli are thought to play an important role in flight stabilization, this is less likely with hawkmoth ocelli, which are small (approx. 100 μm diameter) and largely buried in scales near the base of the antennae. The importance of vision to hovering is reflected in the stabilization response of hovering moths to purely visual perturbations (Kern & Varjú 1998), even in the absence of actual wind or acceleration.
Night vision and the nocturnal behaviours it enables offer an insect the potential to forage in an environment with fewer predators and competitors. Hovering offers the ability to visit more flowers in a given period of time, and possibly increases nectar intake substantially. By sacrificing spatial acuity, the nocturnal A. atropos maintains a motion pathway with high temporal frequency responses compared with other dim-light moths, but cannot hover. The hoverer M. sexta maintains a motion pathway with higher spatial and temporal acuity, but does not forage at the dimmest light levels. The competing demands that these behaviours place on the visual system make D. elpenor a remarkable example for achieving both at once.
Acknowledgements
We would like to acknowledge the useful comments of the anonymous reviewers, as well as the support of and valuable discussions with Dr Tom Daniel and Dr Mark Frye. We thank Dr Alumt Kelber for donation of pupae. J.C.T. was supported by an NIH Neuroscience Training Grant and E.J.W. is grateful to the ongoing support of the Swedish Research Council and the US Air Force Office of Scientific Research.
References
- Budick S. A., Reiser M. B., Dickinson M. H.2007The role of visual and mechanosensory cues in structuring forward flight in Drosophila melanogaster. J. Exp. Biol. 210, 4092–4103 (doi:10.1242/jeb.006502) [DOI] [PubMed] [Google Scholar]
- Chow D. M., Frye M. A.2008Context-dependent olfactory enhancement of optomotor flight control in Drosophila. J. Exp. Biol. 211, 2478–2485 (doi:10.1242/jeb.018879) [DOI] [PubMed] [Google Scholar]
- Collett T. S.1971Visual neurones for tracking moving targets. Nature 232, 127–130 (doi:10.1038/232127a0) [DOI] [PubMed] [Google Scholar]
- Dreisig H.1997Why do some nectar foragers perch and others hover while probing flowers? Evol. Ecol. 11, 543–555 (doi:10.1007/s10682-997-1510-5) [Google Scholar]
- Dror R. O., O'Carroll D. C., Laughlin S. B.2001Accuracy of velocity estimation by Reichardt correlators. J. Opt. Soc. Am. A 18, 241–252 (doi:10.1364/JOSAA.18.000241) [DOI] [PubMed] [Google Scholar]
- Ellington C. P.1984aThe aerodynamics of hovering insect flight. III. Kinematics. Phil. Trans. R. Soc. Lond. B 305, 41–78 (doi:10.1098/rstb.1984.0051) [Google Scholar]
- Ellington C. P.1984bThe aerodynamics of hovering insect flight. VI. Lift and power requirements. Phil. Trans. R. Soc. Lond. B 305, 145–181 (doi:10.1098/rstb.1984.0054) [Google Scholar]
- Farina W. M., Varjú D., Zhou Y.1994The regulation of distance to dummy flowers during hovering flight in the hawk moth Macroglossum stellatarum. J. Comp. Physiol. A 174, 239–247 (doi:10.1007/BF00193790) [Google Scholar]
- Frederiksen R., Wcislo W., Warrant E.2008Visual reliability and information rate in the retina of a nocturnal bee. Curr. Biol. 18, 349–353 (doi:10.1016/j.cub.2008.01.057) [DOI] [PubMed] [Google Scholar]
- Goyret J., Raguso R. A.2006The role of mechanosensory input in flower handling efficiency and learning by Manduca sexta. J. Exp. Biol. 209, 1585–1593 (doi:10.1242/jeb.02169) [DOI] [PubMed] [Google Scholar]
- Haddock S. H., Heine J. N.2005Scientific blue-water diving La Jolla, CA: California Sea Grant College Program [Google Scholar]
- Heimonen K., Salmela I., Kontiokari P., Weckstrom M.2006Large functional variability in cockroach photoreceptors: optimization to low light levels. J. Neurosci. 26, 13 454–13 462 (doi:10.1523/JNEUROSCI.3767-06.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelber A., Balkenius A., Warrant E. J.2002Scotopic colour vision in nocturnal hawkmoths. Nature 419, 922–925 (doi:10.1038/nature01065) [DOI] [PubMed] [Google Scholar]
- Kelber A., Warrant E. J., Pfaff M., Wallen R., Theobald J. C., Wcislo W. T., Raguso R. A.2006Light intensity limits foraging activity in nocturnal and crepuscular bees. Behav. Ecol. 17, 63–72 (doi:10.1093/beheco/arj001) [Google Scholar]
- Kern R., Varjú D.1998Visual position stabilization in the hummingbird hawk moth, Macroglossum stellatarum L. I. Behavioural analysis. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 182, 225–237 (doi:10.1007/s003590050173) [DOI] [PubMed] [Google Scholar]
- Kirschfeld K.1976The resolution of lens and compound eyes. In Neural principles in vision (eds Zettler F., Weiler R., Alkon D. L.), pp. 354–370 Berlin, Germany: Springer Verlag [Google Scholar]
- Kitching I. J.2003Phylogeny of the death's head hawkmoths, Acherontia [Laspeyres], and related genera (Lepidoptera: Sphingidae: Sphinginae: Acherontiini). Syst. Entomol. 28, 71–88 (doi:10.1046/j.1365-3113.2003.00199.x) [Google Scholar]
- Kitching I. J., Cadiou J. M.2000Hawkmoths of the world: an annotated and illustrated revisionary checklist (Lepidoptera: Sphingidae) London, UK and Ithaca, NY: Natural History Museum/Cornell University Press [Google Scholar]
- Land M. F.1981Optics and vision in invertebrates. In Handbook of sensory physiology (ed. Autrum H.), pp. 472–592 Berlin, Germany: Springer [Google Scholar]
- Land M. F., Nilsson D.2002Animal eyes Oxford, UK: Oxford University Press [Google Scholar]
- Laughlin S. B.2001Energy as a constraint on the coding and processing of sensory information. Curr. Opin. Neurobiol. 11, 475–480 (doi:10.1016/S0959-4388(00)00237-3) [DOI] [PubMed] [Google Scholar]
- Laughlin S. B., Weckstrom M.1993Fast and slow photoreceptors—a comparative study of the functional diversity of coding and conductances in the Diptera. J. Comp. Physiol. A 172, 593–609 (doi:10.1007/BF00213682) [Google Scholar]
- McIntyre P., Caveney S.1998Superposition optics and the time of flight in onitine dung beetles. J. Comp. Physiol. A 183, 45–60 (doi:10.1007/s003590050233) [Google Scholar]
- Murlis J., Elkinton J. S., Carde R. T.1992Odor plumes and how insects use them. Annu. Rev. Entomol. 37, 505–532 (doi:10.1146/annurev.en.37.010192.002445) [Google Scholar]
- O'Carroll D. C., Bidweii N. J., Laughlin S. B., Warrant E. J.1996Insect motion detectors matched to visual ecology. Nature 382, 63–66 (doi:10.1038/382063a0) [DOI] [PubMed] [Google Scholar]
- O'Carroll D. C., Laughlin S. B., Bidwell N. J., Harris R. A.1997Spatio-temporal properties of motion detectors matched to low image velocities in hovering insects. Vis. Res. 37, 3427–3439 (doi:10.1016/S0042-6989(97)00170-3) [DOI] [PubMed] [Google Scholar]
- Parsons M. M., Krapp H. G., Laughlin S. B.2006A motion-sensitive neurone responds to signals from the two visual systems of the blowfly, the compound eyes and ocelli. J. Exp. Biol. 209, 4464–4474 (doi:10.1242/jeb.02560) [DOI] [PubMed] [Google Scholar]
- Pick B., Buchner E.1979Visual movement detection under light-and dark-adaptation in the fly, Musca domestica. J. Comp. Physiol. A 134, 45–54 (doi:10.1007/BF00610276) [Google Scholar]
- Pittaway A. R.1993The hawkmoths of the western Palaearctic Colchester, UK: Harley Books [Google Scholar]
- Pringle J. W. S.1948The gyroscopic mechanism of the halteres of Diptera. Phil. Trans. R. Soc. Lond. B 233, 347–384 (doi:10.1098/rstb.1948.0007) [Google Scholar]
- Sane S. P., Dieudonne A., Willis M. A., Daniel T. L.2007Antennal mechanosensors mediate flight control in moths. Science 315, 863–866 (doi:10.1126/science.1133598) [DOI] [PubMed] [Google Scholar]
- Snyder A. W., Stavenga D. G., Laughlin S. B.1977aSpatial information capacity of compound eyes. J. Comp. Physiol. A 116, 183–207 (doi:10.1007/BF00605402) [Google Scholar]
- Snyder A. W., Laughlin S. B., Stavenga D. G.1977bInformation capacity of eyes. Vis. Res. 17, 1163–1175 (doi:10.1016/0042-6989(77)90151-1) [DOI] [PubMed] [Google Scholar]
- Sprayberry J. D., Daniel T. L.2006Flower tracking in hawkmoths: behavior and energetics. J. Exp. Biol. 210, 37–45 (doi:10.1242/jeb.02616) [DOI] [PubMed] [Google Scholar]
- Straw A. D., Rainsford T., O'Carroll D. C.2008Contrast sensitivity of insect motion detectors to natural images. J. Vis. 8, 1–9 (doi:10.1167/8.3.32) [DOI] [PubMed] [Google Scholar]
- Van Hateren J. H.1993Spatiotemporal contrast sensitivity of early vision. Vis. Res. 33, 257 (doi:10.1016/0042-6989(93)90163-Q) [DOI] [PubMed] [Google Scholar]
- Warrant E. J.1999Seeing better at night: life style, eye design and the optimum strategy of spatial and temporal summation. Vis. Res. 39, 1611–1630 (doi:10.1016/S0042-6989(98)00262-4) [DOI] [PubMed] [Google Scholar]
- Warrant E. J.2004Vision in the dimmest habitats on earth. J. Comp. Physiol. A 190, 765–789 (doi:10.1007/s00359-004-0546-z) [DOI] [PubMed] [Google Scholar]
- Warrant E. J.2008Nocturnal vision. In The senses: a comprehensive reference (eds Albright T. L., Masland R. H.), pp. 53–86 Oxford, UK: Academic Press [Google Scholar]
- Warrant E. J., McIntyre P. D.1992The trade-off between resolution and sensitivity in compound eyes. In Nonlinear vision (eds Pinter R. B., Nabet B.), pp. 391–421 Boca Raton, FL: CRC Press Inc [Google Scholar]
- Warrant E. J., Bartsch K., Günther C.1999Physiological optics in the hummingbird hawkmoth: a compound eye without ommatidia. J. Exp. Biol. 202, 497–511 [DOI] [PubMed] [Google Scholar]
- Warrant E. J., Kelber A., Gislén A., Greiner B., Ribi W., Wcislo W. T.2004Nocturnal vision and landmark orientation in a tropical halictid bee. Curr. Biol. 14, 1309–1318 (doi:10.1016/j.cub.2004.07.057) [DOI] [PubMed] [Google Scholar]
- Wcislo W. T., Arneson L., Roesch K., Gonzalez V., Smith A., Fernandez H.2004The evolution of nocturnal behaviour in sweat bees, Megalopta genalis and M. ecuadoria (Hymenoptera: Halictidae): an escape from competitors and enemies? Biol. J. Linn. Soc. 83, 377–387 (doi:10.1111/j.1095-8312.2004.00399.x) [Google Scholar]
- Wicklein M., Strausfeld N. J.2000Organization and significance of neurons that detect change of visual depth in the hawk moth Manduca sexta. J. Comp. Neurol. 424, 356–376 (doi:10.1002/1096-9861(20000821)424:2<356::AID-CNE12>3.0.CO;2-T) [PubMed] [Google Scholar]
- Willmott A., Ellington C.1997The mechanics of flight in the hawkmoth Manduca sexta. II. Aerodynamic consequences of kinematic and morphological variation. J. Exp. Biol. 200, 2723–2745 [DOI] [PubMed] [Google Scholar]