Abstract
Aardonyx celestae gen. et sp. nov. is described from the upper Elliot Formation (Early Jurassic) of South Africa. It can be diagnosed by autapomorphies of the skull, particularly the jaws, cervical column, forearm and pes. It is found to be the sister group of a clade of obligatory quadrupedal sauropodomorphs (Melanorosaurus + Sauropoda) and thus lies at the heart of the basal sauropodomorph–sauropod transition. The narrow jaws of A. celestae retain a pointed symphysis but appear to have lacked fleshy cheeks. Broad, U-shaped jaws were previously thought to have evolved prior to the loss of gape-restricting cheeks. However, the narrow jaws of A. celestae retain a pointed symphysis but appear to have lacked fleshy cheeks, demonstrating unappreciated homoplasy in the evolution of the sauropod bulk-browsing apparatus. The limbs of A. celestae indicate that it retained a habitual bipedal gait although incipient characters associated with the pronation of the manus and the adoption of a quadrupedal gait are evident through geometric morphometric analysis (using thin-plate splines) of the ulna and femur. Cursorial ability appears to have been reduced and the weight bearing axis of the pes shifted to a medial, entaxonic position, falsifying the hypothesis that entaxony evolved in sauropods only after an obligate quadrupedal gait had been adopted.
Keywords: sauropod, sauropodomorph, Aardonyx celestae, bulk browsing, quadrupedal gait
1. Introduction
Eusauropod dinosaurs possess a highly specialized set of skeletal adaptations related to their gigantic size, obligate quadrupedalism, graviportal locomotion and strictly herbivorous diets (Upchurch et al. 2004 and references therein). Indeed, the evolution of sauropods from earlier basal sauropodomorphs is perhaps the most extreme morphological transformation to have occurred in early dinosaur evolution. The nature of this transition has been obscure but new discoveries over the last dozen years have shed much light upon it. Cladistic analyses of sauropod relationships have identified plesiomorphic members of the Sauropoda and provided an outline of the sequence in which their various specializations were acquired (Upchurch 1998; Wilson & Sereno 1998; Wilson 2002; Upchurch et al. 2004). Biomechanical studies have also begun to unravel the functional significance of some of these characters (Bonnan 2003; Carrano 2005). The first Triassic sauropods have also come to light in the last decade, revealing some of the morphology of the basal-most members of the clade (Buffetaut et al. 2000; Yates & Kitching 2003). There has also been a flurry of cladistic analyses on the wider sauropodomorph clade, putting Sauropoda into its wider context (Benton et al. 2000; Yates 2003, 2004, 2007; Upchurch et al. 2004, 2007a). While it is true that these analyses have produced widely divergent results, there is now general agreement that basal sauropodomorphs (traditionally ‘prosauropods’) are paraphyletic to some extent with respect to Sauropoda. Lastly, detailed descriptions of advanced near-sauropod sauropodomorphs have elucidated the morphology of the closest sauropod ancestors (Bonnan & Yates 2007; Kutty et al. 2007; Pol & Powell 2007; Upchurch et al. 2007b; Yates 2007). Despite all this research, many aspects of the transition remain unknown owing to a combination of uncertainty surrounding the precise phylogenetic relationships of basal sauropodomorphs, gaps in the phylogenetic sequence and the incompleteness of most of the taxa that are known from this transition.
Here, we report on Aardonyx celestae gen. et sp. nov., a sauropodomorph that lies in the heart of the basal sauropodomorph-sauropod transition. Aardonyx appears to be the closest known sister group to the clade of obligatory quadrupedal sauropodomorphs to retain facultative, if not habitual, bipedalism.
2. Systematic palaeontology
Sauropodomorpha Von Huene, 1932
Anchisauria Galton and Upchurch, 2004
Aardonyx celestae gen. et sp. nov
(a). Holotype
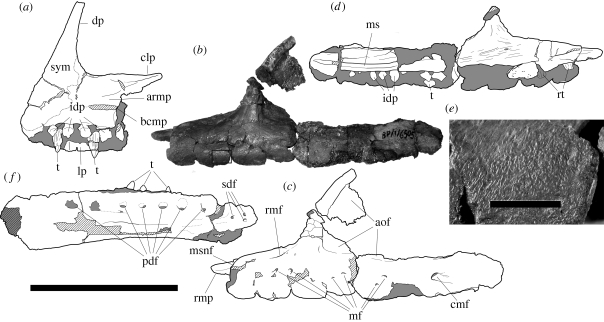
Rostral half of the left maxilla (BP/1/6254) (figure 1b–e). A non-overlapping, weathered, caudal portion of a left maxilla (BP/1/6505) was found about a metre from the holotype, and may well represent the same bone as the holotype.
Figure 1.
Holotype and other jaw elements of Aardonyx celestae gen. et sp. nov. (a) Right premaxilla (BP/1/6584) in medial view. (b–d) Holotype left maxilla (BP/1/6254) and caudal fragment (BP/1/6505) in (b,c) lateral and (d) medial views. Box indicates the area enlarged in (e). (e) Close-up of lateral supra-alveolar surface of the holotype maxilla (BP/1/6254). (f) Right dentary (BP/1/6334) in lateral view. Hatched areas represent broken bone surfaces, grey areas indicate areas of matrix. Abbreviations: aof, antorbital fossa; armp, articulation surface for the rostromedial process of the maxilla; bcmp, base of the caudomedial process of the premaxilla; clp, caudolateral process of the premaxilla; cmf, caudal maxillary foramen; dp, dorsal process of the premaxilla; idp, interdental plate; lp, lateral plate; mf, maxillary foramen; ms, medial sulcus of the maxilla; msnf, maxillary margin of the subnarial foramen; pdf, primary dentary foramina; rmf, rostral maxillary foramen; rmp, rostromedial process of the maxilla; rt, replacement tooth; sdf, secondary dentary foramina; sym, symphyseal surface; t, tooth. Scale bar 100 mm in (a–d,f), scale bar in (e), 5 mm.
(b). Type locality and horizon
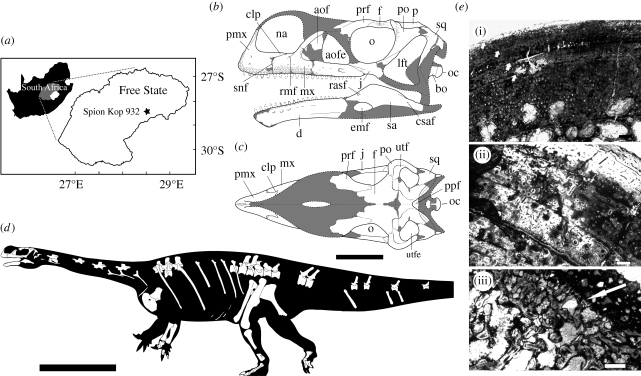
Marc's Quarry (MQ) bone bed on the farm Spion Kop 932, Senekal District, Free State, South Africa (figure 2a). The bone bed is situated in the Early Jurassic upper Elliot Formation (Bordy et al. 2004).
Figure 2.
(a) Map of South Africa showing the location of Spion Kop 932. (b,c) Reconstruction of the skull of Aardonyx celestae gen. et sp. nov. in (b) lateral and (c) dorsal views. (d) Reconstruction of the skeleton of Aardonyx celestae gen. et sp. nov. scaled to the size of the smaller individual. (e) Histological sections of bones from Aardonyx celestae gen. et sp. nov. (i) shows a transverse section through a rib. The cortical bone consists of well-vascularized fibro-lamellar bone tissue with distinct lines of arrested growth (arrows) and several secondarily enlarged erosion cavities. (ii) shows a transverse section of a scapula fragment showing the zonal nature of the compacta. (iii) shows a longitudinal section of a scapular fragment with calcified cartilage at the articular edge of the bone (arrowed). Abbreviations: aof, antorbital fossa; aofe, antorbital fenestra; bo, basioccipital; clp, caudolateral process; csaf, caudal surangular foramen; d, dentary; emf, external mandibular fenestra; f, frontal; j, jugal; ltf, laterotemporal fenestra; mx, maxilla; na, external naris; o, orbit; oc, occipital condyle; p, parietal; pmx, premaxilla; po, postorbital; rmf, rostral maxillary foramen; rsaf, rostral surangular foramen; sa, surangular; sq, squamosal; utf, upper temporal fossa; utfe, upper temporal fenestra. Scale bars, 100 mm in (b,c), 1 m in (d) and 500 µm in (e).
(c). Referred specimens
A large number of disarticulated bones from the type locality, including skull elements, mandibular elements, vertebrae from the cervical, dorsal, sacral and caudal series, cervical ribs, dorsal ribs, gastralia, chevrons, pectoral girdle elements, pelvic girdle elements and bones of both the fore- and hind limbs, manus and pes. All of these bones are from the type quarry and seem to derive from two immature individuals, the smaller with linear dimensions of the postcranial elements that are about 85 per cent of the larger individual.
The referral of the numerous disarticulated elements from MQ to Aardonyx is justified by a taphonomic study of the site (see the electronic supplementary material).
(d). Etymology
Aardonyx from aard (Afrikaans for ‘Earth’) and onyx (Greek for ‘claw’), gender is masculine; celestae for Celeste Yates who prepared many of the bones. Genus name refers to the thick hematite encrustation of many of the bones, particularly the ungual phalanges, in the type quarry.
(e). Diagnosis
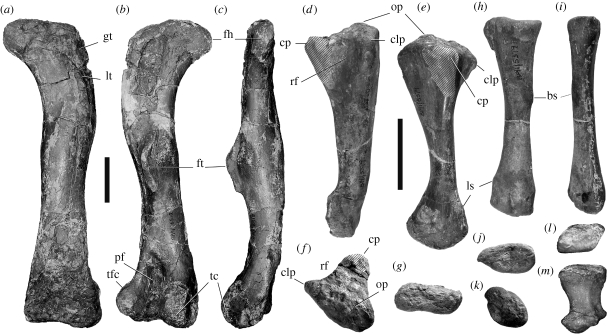
A sauropodomorph with the following autapomorphies: five premaxillary teeth (convergent in Plateosaurus) (figure 1a); a band of dense, fine pits and small foramina along the lower half of the lateral surface of the maxilla (figure 1e); reduced lateral maxillary neurovascular foramina rostral to the large caudally facing foramen at the caudal end of the maxilla (middle foramina are <6% of the depth of the maxilla caudal to the antorbital fossa) (figure 1c); an elongate rostral ramus of the maxilla combined with a steep dorsal process of the premaxilla to produce an enlarged external naris (area at least subequal to that of the orbit) (figure 2b); a well-developed longitudinal sulcus on the medial side of the caudal maxillary ramus (figure 1d); reduced cervical diapophyses that remain as low tubercles, with a concomitant absence of the diapophyseal laminae, along the full length of the cervical series; large, rugose biceps scar (maximum diameter 13% of the length of the radius) on the craniomedial surface of the shaft of the radius (figure 3h,i); exceptionally broad and flat proximal end of metatarsal IV (transverse width is 2.9 times greater than the extensor–flexor depth); distal end of metatarsal IV with a strongly laterally flared caudolateral corner.
Figure 3.
Limb elements of Aardonyx celestae gen. et. sp. nov. (a–c) Left femur (BP/1/6510) of small individual in (a) cranial, (b) caudal and (c) medial views. (d–g) Left ulna (BP/1/5379c) of large individual in (d) craniolateral, (e) cranial, (f) proximal and (g) distal views (cranial direction to the top in proximal and distal views). (h–k) Left radius (BP/1/5379d) of large individual in (h) medial, (i) cranial, (j) proximal and (k) distal views (cranial direction is to the right for proximal and distal views). (l,m) Right metatarsal I (BP/1/6602) of the small individual in (l) proximal and (m) cranial views. Abbreviations: bs, biceps scar; clp, craniolateral process of the ulna; cp, cranial process of the ulna; ct, cranial trochanter; ft, fourth trochanter; fh, femoral head; gt, greater trochanter; ls, ligament scar; op, olecranon process; pf, popliteal fossa; rf, radial fossa; tc, tibial condyle; tfc, tibiofibular crest. Hatching represents areas of plaster reconstruction. Scale bars, 100 mm, with the left bar pertaining to (a–c) and the right bar to (d–m).
In addition to these autapomorphies, Aardonyx can be further distinguished from members of the quadrupedal sauropodomorph clade, such as Melanorosaurus and Antetonitrus by an absence of an inflection in the profile of the snout at the base of the nasal process of the premaxilla; a slender ventral ramus of the squamosal (basal width of the ramus is 33% of its length); a small, poorly developed craniolateral process at the proximal end of the ulna; a sacrum consisting of just three vertebrae; a sinuous lateral margin of the femoral shaft; femoral shaft with a subcircular cross-section; a cranial trochanter that is placed well away from the lateral margin of the femur in cranial view and is not visible when the femur is viewed caudally. It can be distinguished from more primitive near-sauropod sauropodomorphs such as Jingshanosaurus, Anchisaurus and Yunnanosaurus by its broad subtriangular ascending ramus of the maxilla, presence of labial plates on the premaxilla, maxilla and dentary, transversely broad prefrontal, absence of a caudal lateral ridge on the dentary, taller mid-dorsal neural spines, a less strongly developed distal swelling of the pubis, a descending caudolateral process of the distal end of the tibia that fails to extend to the level of the cranial lateral corner of the distal articular surface, the robust metatarsal I with a proximal end that is about 75 per cent of the total length, and the stout pedal phalanges which are not longer than their proximal transverse width. For a description of the Aardonyx remains, see the electronic supplementary material.
(f). Osteohistology and ontogenetic age of the material
Thin sections of a fragment of a rib and scapula from Aardonyx were prepared using the methodology described by Chinsamy-Turan (2005) (figure 2e). The cortices of both bones show zonal bone tissue: highly vascularized fibrolamellar bone within zones, alternating with distinct lines of arrested growth. The rib fragment displays five growth rings, whereas the scapular fragment has seven. Neither bone shows any peripheral rest lines to suggest that appositional growth had stopped, therefore indicating skeletally immature individuals. The earliest line of arrested growth in the rib is followed by the widest zone, indicating that it was probably laid down in the rapid growth phase of early ontogeny, suggesting that few, if any, growth lines had been obliterated owing to medullary expansion. That the individual(s) were still growing at the time of death is supported by the presence of calcified cartilage at the articular end of the scapula, indicating continued growth in bone length (Horner et al. 2001; Chinsamy-Turan 2005). In conclusion, the histological analysis suggests that the individual(s) sampled were actively growing and possibly less than 10 years old at the time of death.
3. Phylogenetics
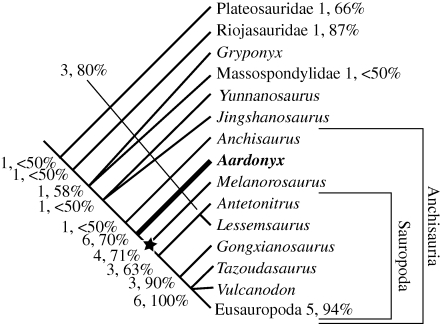
Aardonyx was added to modified versions of two recent, comprehensive cladistic analyses of basal sauropodomorph relationships (Upchurch et al. 2007a; Yates 2007). In both cases (figure 4, and the electronic supplementary material), it was found to lie at the heart of the basal sauropodomorph-sauropod transition as the closest outgroup to the clade containing what we interpret to be the obligatory quadrupedal sauropodomorphs (Melanorosaurus + Sauropoda). As such, it is an important morphological intermediate that sheds much light on the nature of this transition. Derived traits supporting this relationship include labial alveolar margins of the premaxilla, maxilla and dentary forming lateral plates (figure 1b); reversal to mid-cervical neural spines that are less than twice as long as high; hyposphenes in the dorsal vertebrae as deep as the neural canal; height of the middle dorsal neural spines greater than the length of the base; reversal to less than 60° of ventrolateral twisting of the first phalanx of manual digit I; proximal tip of the cranial trochanter distal to the femoral head (convergent with many basal sauropomorphs) (figure 3a); fourth trochanter positioned over the midlength of the femur (figure 3b,c); a robust metatarsal I with a minimum transverse midshaft diameter that exceeds that of metatarsal II (figure 3m); at least the distal non-terminal pedal phalanges are wider than long; ungual of pedal digit I is longer than the first phalanx of pedal digit I; adult femur length that exceeds 600 mm.
Figure 4.
Condensed cladogram based on the strict consensus of 28 most parsimonious trees (tree length = 1119) obtained from a cladistic analysis of a modified version of the Yates (2007) matrix (353 characters; see the electronic supplementary material for details) after the a priori removal of the poorly known, and unstable taxa: Plateosaurus (=Gresslyosaurus) ingens, Camelotia, Blikanasaurus and Isanosaurus (leaving 44 active taxa in the analysis). Only the plateosaurian part of the tree is shown here. Named suprageneric taxa are collapsed into single terminals to save space: Plateosauridae contains Unaysaurus, Plateosaurus gracilis and Plateosaurus engelhardti; Riojasauridae contains Riojasaurus and Eucnemesaurus; Massospondylidae contains Massospondylus, Coloradisaurus and Lufengosaurus; Eusauropoda contains Shunosaurus, Omeisaurus, Mamenchisaurus, Barapasaurus, Patagosaurus, Cetiosaurus and Neosauropoda. Bold numbers given at each node are decay indices, the percentages are bootstrap support values. The star marks the basal node of the quadrupedal clade.
Although the relationships among non-sauropods remain weak in the matrix based on Upchurch et al. (2007a), the clade of Aardonyx plus the quadrupedal sauropodomorphs is robust in the modified version of Yates' (2007) matrix, once the poorly known, unstable taxa (Plateosaurus (=Gresslyosaurus) ingens, Camelotia and Isanosaurus) are removed (figure 4).
4. The evolution of sauropod bulk browsing
Eusauropods show a complex of derived character states that appear to be adaptations towards a bulk-browsing mode of feeding. Primarily, this complex consists of three characteristics: the development of lateral plates along the alveolar margins of tooth-bearing bones that brace the lingual sides of the teeth against bucco-lingual forces during foliage stripping; broad, U-shaped jaws to allow a wider bite; and loss of fleshy cheeks to increase gape (Upchurch & Barrett 2000; Upchurch et al. 2007b).
Aardonyx shows plesiomorphic, narrow, V-shaped jaws combined with the derived absence of a lateral ridge at the caudal end of the dentary (figure 1f). The latter feature is also absent in all known sauropods, except Chinshakiangosaurus (Upchurch et al. 2007b). It is thought to be related to the loss of fleshy cheeks in order to facilitate a wider gape for bulk browsing (Upchurch et al. 2007b). Further evidence that Aardonyx lacked extensive fleshy cheeks can be gleaned from the lateral neurovascular foramina of the maxilla. These openings are smaller than in those of most other basal sauropodomorphs (figure 1c). This indicates that there was a reduction in the blood supply to the buccal tissues which, in turn, suggests the loss, or reduction, of fleshy cheeks. The dense pitting of the labial alveolar margins of the premaxilla, maxilla and dentary is interesting in this respect since pits of similar size and density are also found along the alveolar margins of extant crocodylians. It is possible that, in life, the gum line of Aardonyx was lined with tightly adherent cornified tissue like those of extant crocodylians. However, the number of lateral neurovascular foramina on the maxilla and dentary (no more than 11 foramina per bone) suggests otherwise. All modern tetrapods with similar low numbers of lateral neurovascular foramina possess an extra-oral soft-tissue covering of the teeth (Morhardt et al. 2009). Thus, it is probable that even if cheeks were not present, the living Aardonyx sported thin, lizard-like lips.
The combination of narrowly pointed but cheekless jaws is the opposite of the condition seen in the Chinese basal sauropod Chinshakiangosaurus, where the jaws are broad and U-shaped but retain a well-developed caudal lateral dentary ridge (Upchurch et al. 2007b). Thus, a wider, cheekless gape may have evolved twice in Sauropodomorpha: once in Aardonyx and once in sauropods more derived than Chinshakiangosaurus.
5. The evolution of obligate quadrupedalism in sauropodomorphs
The clade of Melanorosaurus + Sauropoda would appear to be diagnosed by habitual, if not obligate, quadrupedalism. This interpretation is supported by modifications of both the fore- and hindlimbs of members of this clade. These are as follows.
Increase of the relative length of the forearm relative to the hindlimb (humerus: femur ratio >0.8) in large post-hatching individuals. Lessening the discrepancy between fore- and hindlimb length is clearly advantageous to a quadruped. Sauropodomorphs basal to this clade have a humerus : femur ratio that is less than 0.8, and usually less than 0.7 (Cooper 1981), in large post-hatching individuals. Note that hatchlings and very young basal sauropodomorphs had high humerus : femur ratios but were also obligate quadrupeds (Reisz et al. 2005).
Development of a large craniolateral process at the proximal end of the ulna. This process defines a deep cranially facing radial fossa that holds the radius in a medially shifted position, so that the distal end of the radius lays craniomedial to the ulna. This pronates the manus and brings the direction of flexion–extension of the wrist closer to parallel with the direction of travel (Bonnan 2003).
Straightening of the femoral shaft. This is particularly apparent along the proximal lateral margin in cranial view (figure 3a,b). In basal sauropodomorphs, this margin is markedly convex, whereas it is straight in Melanorosaurus and basal sauropods. The loss of femoral sinuosity is associated with the development of a more columnar stance with reduced limb excursions during locomotion, i.e. a trend towards graviportalism. Only quadrupedal dinosaur clades have evolved graviportalism (e.g. Sauropoda, Stegosauria and Nodosauridae), indicating that the trend towards it in the clade of Melanorosaurus + Sauropoda was probably correlated with quadrupedalism.
It should be noted that in basal members of this clade (e.g. Melanorosaurus and Antetonitrus), the manus still retained some degree of functionality for non-locomotor purposes, including an offset and mobile pollex with some grasping ability (Yates & Kitching 2003; Bonnan & Yates 2007). As a consequence, it has been suggested that these features imply facultative bipedalism (Carrano 2005) but they may simply represent plesiomorphic retentions. In any case, crude grasping ability need not imply bipedalism because their hands could have been employed singly while the animal was stationary.
The quadrupedal clade is also diagnosed by an increase in the number of sacral vertebrae (from three to at least four) and the development of an eccentric femoral shaft (one where the mediolateral dimension of the cross-section exceeds the craniocaudal dimension) to counter increased mediolateral forces. Neither of these is necessarily an adaptation to quadrupedalism, although both may be adaptations to support an increasing gut volume and mass, relative to body size, which may have been facilitated by quadrupedalism. Lastly, the quadrupedal clade is diagnosed by an apparent lateral shift in the position of the cranial trochanter relative to the femoral head, such that it is visible in caudal view. The reason for this shift is unclear but it does indicate that the pelvic-femoral musculature was remodelled at this node.
Aardonyx lacks these specializations and was probably bipedal. In particular, the humerus : femur ratio is approximately 72 per cent in the smaller individual (humerus length is estimated from the radius length). The radius and ulna of Aardonyx clearly show that it could not actively pronate its manus to any great extent. The shaft of the radius is nearly straight with a slightly medial curvature, and the radial head is ovate, preventing its rotation about the ulna (figure 3h–k). Nevertheless, the ulna associated with the radius shares some similarities with those of obligatory quadrupedal sauropodomorphs. The proximal end possesses an incipient craniolateral process which produces a subtle version of the Y-shaped outline that is more fully developed in the quadrupedal clade (Bonnan & Yates 2007) (figure 3f). There is a shallow radial fossa, which cradles the radius craniolaterally. In articulation, the position of the radius in relation to the ulna is shifted slightly cranially owing to the presence of the incipient craniolateral process. This is similar to, but less well-developed than, the more derived cranial and medial orientation of the radius in Melanorosaurus and sauropods, but is insufficient to translate into a significant excursion of the distal end of the radius (see the electronic supplementary material). The distal ends of the radius and the ulna contain rugose and scarred areas that may be associated with ligaments (Bonnan 2003) (figure 3e,h). The presence of these features suggests that these elements were bound distally, precluding any active pronation or supination of the manus.
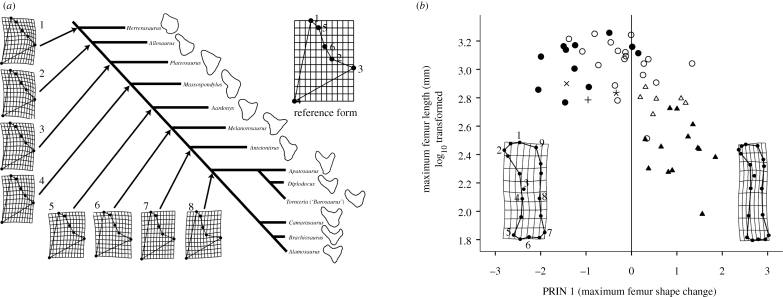
A geometric morphometric analysis of proximal ulna morphology in saurischian dinosaurs further supports our inferences. Using the thin-plate splines suite of programmes (Rohlf 2005), we digitized the regions of the craniomedial, craniolateral and olecranon processes as well as regions which outline the proximal shape of the radial fossa in selected saurischian ulnae. The program TPSTree was then used to predict the shape of the hypothetical common ancestral ulna at each node in a simplified cladogram composed of our selected taxa. Landmark coordinates in each specimen were scaled, rotated and aligned and compared against a grand mean form in the sequence predicted by the phylogenetic pattern. This generated a suite of dependent shape variables known as partial warps used to compute deformation grids which predict how and where the ulna changed shape proximally at each hypothetical common ancestor.
Although these data are exploratory and cannot be said to show statistically significant differences (Zelditch et al. 2004; Bonnan 2007), nevertheless, we are intrigued that the ulna of Aardonyx is the first in the sequence to show a noticeable craniolateral process (landmark 3) and medially shifted radial fossa (landmarks 2, 5, 6) (figure 5a).
Figure 5.
Geometric morphometric analysis of proximal ulna and femur shape in selected saurischian dinosaurs. (a) Proximal ulna shape. The cladogram is based on the topology from figure 4 with additional neosauropod resolution from Upchurch et al. (2004). The proximal outline of the ulna of each taxon is shown on the right side of the cladogram. The reference form is the scaled, rotated and aligned average of all specimens in the sample, and the deformation grids along the cladogram show how the proximal ulna differs from the reference form at each node. The deformation grids represent the predicted proximal ulna shape of the hypothetical common ancestor at each node: (1) Saurischia; (2) Eusaurischia; (3) Sauropodomorpha; (4) Massospondylus + Anchisauria; (5) Aardonyx + quadrupedal clade; (6) the quadrupedal clade: Melanorosaurus + Sauropoda; (7) Sauropoda; and (8) Neosauropoda. Note the change in deflection of the craniolateral process (landmarks 2,3) at node (5). (b) Sauropodomorph femur shape versus size. Note that the femur of Aardonyx is nestled within sauropods on the graph, and that it plots within close proximity to both the basal sauropod Antetonitrus and sauropod sister taxon Melanorosaurus. Triangles are basal sauropodomorphs, circles are sauropods, the × is Antetonitrus, the + is Melanorosaurus and Aardonyx is represented by the asterix. Filled triangles are Massospondylus, open triangles are Plateosaurus, filled circles are macronarian sauropods and open circles are diplodocoid sauropods. Numbers on the femur deformation grid correspond to anatomical landmarks described in the electronic supplementary material.
Similarly, the femur of Aardonyx is intermediate between the basal sauropodomorph condition (typified by Plateosaurus and Massospondylus) and that of the quadrupedal clade. The shaft retains a convex proximal lateral profile (figure 3a), although the sinuosity of the femur is reduced. The transverse section of the femoral shaft is also subcircular and the cranial trochanter lies in the plesiomorphic position, far from the lateral margin.
Other hindlimb features of Aardonyx indicate that the evolution of quadrupedalism was preceded by the evolution of a slower gait. A geometric morphometric analysis of femur shape (using TPSRelw: Rohlf (2005)) in caudal view of sauropodomorphs and sauropods shows that the femur shape of Aardonyx plots among sauropods, with a relatively straight femoral shaft and, notably, a more distally placed fourth trochanter (figure 5b; statistical details in the electronic supplemental material). A subsequent canonical variance analysis of these data assigned Aardonyx to sauropod femur shape (see the electronic supplementary material). As the main femoral retractor muscle of non-avian saurians, the M. caudofemoralis longus, inserts on the fourth trochanter (Gatesy 1990), a distal shift in this trochanter results in a lower lever ratio, greater mechanical advantage and a decrease in the velocity of femoral retraction as described previously for sauropods (Bonnan 2007).
Lastly, we note that the elements of the foot are relatively short and stout, and that metatarsal I of Aardonyx is remarkably robust in comparison with more basal sauropodomorphs. The maximum midshaft width is 46 per cent of its length whereas this ratio is much lower in more basal sauropodomorphs (Yates 2008). Furthermore, the transverse midshaft width of metatarsal I exceeds that of the other metatarsi, another derived sauropod-like characteristic (Wilson & Sereno 1998). These proportions indicate that the weight bearing axis of Aardonyx had shifted to a more medial, or entaxonic, position than in more basal sauropodomorphs where the weight bearing axis runs through digit III (mesaxony). The loss of mesaxony in Aardonyx is also consistent with the hypothesis that a wider-gauge gait and reduced cursorial ability preceded the evolution of an obligate quadrupedal gait. Previously, the entaxonic pes of eusauropods was thought to have evolved sometime after the divergence of Vulcanodon which has a plesiomorphic, mesaxonic pes (Carrano 2005). However, the hyper-robust first metatarsal of Aardonyx, together with those of the basal sauropods Antetonitrus and Blikanasaurus (Yates 2008), suggests that the mesaxonic pes of Vulcanodon (Cooper 1984) is an evolutionary reversal. Once again, it appears that our incomplete knowledge of the anatomy of near-sauropods and basal sauropods has masked substantial homoplasy associated with the assembly of the eusauropod bauplan.
Acknowledgements
We thank the past and present owners of Spion Kop, Naude Bremmer Sr and Cobus Visser for their hospitality and permission to excavate on their land. We thank Fernando Abdala, Zubair Ali-Jinnah, Natasha Barbolini, Naude Bremmer Jr, Juan Cisneros, Germari DeVilliers, Chalton Dube, Sarah Fowell, John Hancox, Kirat Lalla; Ceri McCrae, Romy Mörsner, Merril Nicolas, Luke Norton, Lucille Pereira, Stephanie Potze, Nkosinathi Sithole, Cecilio Vasconcelos and Celeste Yates for their participation in the fieldwork. We thank Nkosinathi Sithole, Charleton Dube and Celeste Yates for preparation of this material. We acknowledge the support National Geographic Society for funding the fieldwork (CRE no. 7713-04). Support for M.G.B. was given by the Palaeontological Scientific Trust. M.F.B. was supported in part by a Faculty Mentor grant from the College of Arts and Sciences at WIU and a Center for Innovation in Teaching Research, Faculty Research Developmental Activities Award. Page charges were paid for by the College of Arts and Sciences and WIU.
References
- Benton M. J., Juul L., Storrs G. W., Galton P. M.2000Anatomy and systematics of the prosauropod dinosaur Thecodontosaurus antiquus from the Upper Triassic of southwest England. J. Vert. Paleontol. 20, 77–108 (doi:10.1671/0272-4634(2000)020[0077:AASOTP]2.0.CO;2) [Google Scholar]
- Bonnan M. F.2003The evolution of manus shape in sauropod dinosaurs: implications for functional morphology, forelimb orientation, and phylogeny. J. Vert. Paleontol. 23, 595–613 (doi:10.1671/A1108) [Google Scholar]
- Bonnan M. F.2007Linear and geometric morphometric analysis of long bone scaling patterns in Jurassic Neosauropod dinosaurs: their functional and paleobiological implications. Anat. Rec. 290, 1089–1111 (doi:10.1002/ar.20578) [DOI] [PubMed] [Google Scholar]
- Bonnan M. F., Yates A. M.2007A new description of the forelimb of the basal sauropodomorph Melanorosaurus: implications for the evolution of pronation, manus shape and quadrupedalism in sauropod dinosaurs. Spec. Papers Palaeontol. 77, 157–168 [Google Scholar]
- Bordy E. M., Hancox P. J., Rubidge B. S.2004Basin development during the deposition of the Elliot Formation (Late Triassic–Early Jurassic), Karoo Supergroup, South Africa. S. Afr. J. Geol. 107, 395–410 (doi:10.2113/107.3.397) [Google Scholar]
- Buffetaut E., Suteethorn V., Cuny G., Tong H., Le Loeuff J., Khansubha S., Jongautchariyakul S.2000The earliest known sauropod dinosaur. Nature 407, 72–74 (doi:10.1038/35024060) [DOI] [PubMed] [Google Scholar]
- Carrano M. T.2005The evolution of sauropod locomotion: morphological diversity of a secondarily quadrupedal radiation. In The sauropods: evolution and paleobiology (eds Curry Rogers K. A., Wilson J. A.), pp. 229–251 Berkeley, CA: University of California Press [Google Scholar]
- Chinsamy-Turan A.2005The microstructure of dinosaur bone: deciphering biology with fine-scale techniques Baltimore, MD: Johns Hopkins University Press [Google Scholar]
- Cooper M. R.1981The prosauropod dinosaur Massospondylus carinatus Owen from Zimbabwe: its biology, mode of life and phylogenetic significance. Occas. Pap. Natl Mus. Rhodesia B 6, 689–840 [Google Scholar]
- Cooper M. R.1984A reassessment of Vulcanodon karibaensis Raath (Dinosauria: Saurischia) and the origin of the Sauropoda. Palaeontol. Afr. 25, 203–231 [Google Scholar]
- Gatesy S. M.1990Caudofemoral musculature and the evolution of theropod locomotion. Paleobiology 16, 170–186 [Google Scholar]
- Horner J. R., Padian K., de Ricqlès A.2001Comparative osteohistology of some embryonic and perinatal archosaurs: developmental and behavioral implications for dinosaurs. Palaeobiology 27, 39–58 (doi:10.1666/0094-8373(2001)027<0039:COOSEA>2.0.CO;2) [Google Scholar]
- Kutty T. S., Chatterjee S., Galton P. M., Upchurch P.2007Basal sauropodomorphs (Dinosauria: Saurischia) from the Lower Jurassic of India: their anatomy and relationships. J. Paleontol. 81, 1218–1240 (doi:10.1666/04-074.1) [Google Scholar]
- Morhardt A. C., Bonnan M. F., Keillor T.2009Dinosaur smiles: correlating premaxilla, maxilla, and dentary foramina counts with extra-oral structures in amniotes and its implications for dinosaurs. J. Vert. Paleontol. 29(Suppl. 3), 152A.(Abstract) [Google Scholar]
- Pol D., Powell J. E.2007New information on Lessemsaurus sauropoides (Dinosauria: Sauropodomorpha) from the Upper Triassic of Argentina. Spec. Papers Palaeontol. 77, 223–243 [Google Scholar]
- Reisz R. R., Scott D., Sues H.-D., Evans D. C., Raath M. A.2005Embryos of an early prosauropod dinosaur and their evolutionary significance. Science 309, 761–764 (doi:10.1126/science.1114942) [DOI] [PubMed] [Google Scholar]
- Rohlf J. F.2005Thin-plate splines program suite. (http://life.bio.sunysb.edu/morph/) [Google Scholar]
- Upchurch P.1998The phylogenetic relationships of sauropod dinosaurs. Zool. J. Linn. Soc. 124, 43–103 (doi:10.1111/j.1096-3642.1998.tb00569.x) [Google Scholar]
- Upchurch P., Barrett P. M.2000The evolution of sauropod feeding mechanisms. In The evolution of herbivory in terrestrial vertebrates: perspectives from the fossil record (ed. Sues H.-D.), pp. 79–122 Cambridge, UK: Cambridge University Press [Google Scholar]
- Upchurch P., Barrett P. M., Dodson P.2004Sauropoda. In The Dinosauria (eds Weishampel D. B., Dodson P., Osmólska H.), pp. 259–322, 2nd edn Berkeley, CA: University of California Press [Google Scholar]
- Upchurch P., Barrett P. M., Galton P. M.2007aA phylogenetic analysis of basal sauropodomorph relationships: implications for the origin of sauropod dinosaurs. Spec. Papers Palaeontol. 77, 57–90 [Google Scholar]
- Upchurch P., Barrett P. M., Zhao X., Xu X.2007bA re-evaluation of Chinshakiangosaurus chunghoensis Ye vide Dong 1992 (Dinosauria, Sauropodomorpha): implications for cranial evolution in basal sauropod dinosaurs. Geol. Mag. 144, 247–262 (doi:10.1017/S0016756806003062) [Google Scholar]
- Wilson J. A.2002Sauropod dinosaur phylogeny: critique and cladistic analysis. Zool. J. Linn. Soc. 136, 217–276 [Google Scholar]
- Wilson J. A., Sereno P. C.1998Early evolution and higher-level phylogeny of sauropod dinosaurs. Mem. Soc. Vert. Paleontol. 5, 1–68 (doi:10.2307/3889325) [Google Scholar]
- Yates A. M.2003A new species of the primitive dinosaur Thecodontosaurus (Saurischia: Sauropodomorpha) and its implications for the systematics of early dinosaurs. J. Syst. Palaeontol. 1, 1–42 (doi:10.1017/S1477201903001007) [Google Scholar]
- Yates A. M.2004Anchisaurus polyzelus (Hitchcock): the smallest known sauropod dinosaur and the evolution of gigantism among sauropodomorph dinosaurs. Postilla 230, 1–58 [Google Scholar]
- Yates A. M.2007The first complete skull of the Triassic dinosaur Melanorosaurus Haughton (Sauropodomorpha: Anchisauria). Spec. Papers Palaeontol. 77, 9–55 [Google Scholar]
- Yates A. M.2008A second specimen of Blikanasaurus (Dinosauria: Sauropoda) and the biostratigraphy of the lower Elliot Formation. Palaeontol. Afr. 43, 39–43 [Google Scholar]
- Yates A. M., Kitching J. W.2003The earliest known sauropod dinosaur and the first steps towards sauropod locomotion. Proc. R. Soc. Lond. B 270, 1753–1758 (doi:10.1098/rspb.2003.2417) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelditch M. L., Swiderski D. L., Sheets H. D., Fink W. L.2004Geometric morphometrics for biologists: a primer New York, NY: Elsevier Academic Press [Google Scholar]