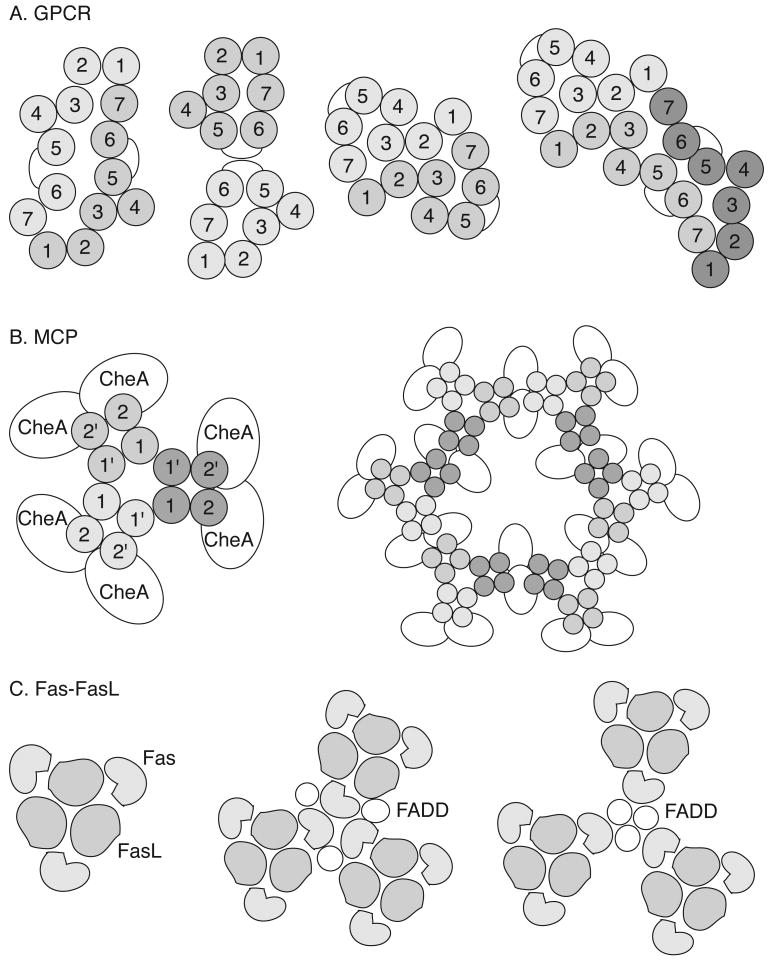
Figure 2.
Proposed multi-receptor assemblies for some receptors. (A) Models of heptahelical GPCR dimers and a trimer based on mutagenesis results and the structure of bacteriorhodopsin. Each numbered circle represents a specific transmembrane helix. A variety of 1-7, 5-6, and 2-3 dimers and multimers have been proposed. (B) Bacterial chemoreceptors (methyl-accepting chemotaxis proteins or MCPs) are dimeric; and these can assemble into a trimer-of-dimers; this trimer of MCP dimers can further interact with signaling proteins, including the kinase CheA. Each MCP passes through the membrane twice (1 and 2) and coiled-coil interactions between these transmembrane domains (1 and 1′) mediate dimerization. A lattice model of MCP organization constructed of six of these trimers-of-dimers and 24 copies of CheA is shown. (C) The Fas-FasL interaction has been modeled using protein interfaces suggested by mutagenesis and crosslinking studies. Both the receptor Fas and its corresponding ligand, FasL, are trimers. The corresponding trimeric complex may be employed as a unit in the lattice stabilized by the adapter protein, FADD. Two models are shown with either Fas- or FADD-centered symmetries. See the text for the appropriate references.