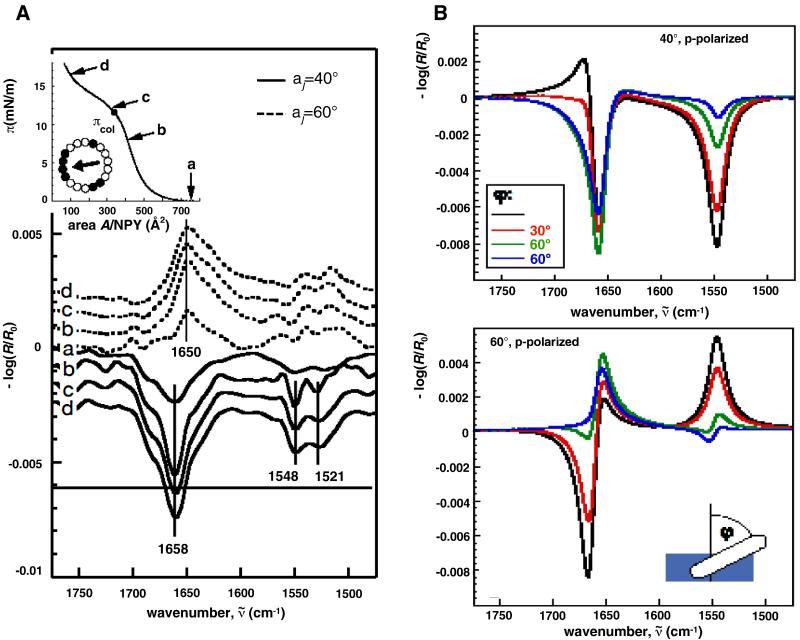
Figure 11.
Determination of the orientation of Neuropeptide Y in a Langmuir film during compression. Characters adjacent to the p-polarized spectra in A), bottom panel correspond to pressures in the π-A isotherm shown in the inset (top left) as follows (a: π =0; b: 8mN/m; c:12 mN/m; d: 16 mN/m). Spectra were acquired for angles of incidence of 40° (solid line) and 60°, (dashed line). Also shown is a helical wheel representation of the peptide α-helix (black: hydrophobic residues; white hydrophilic residues; arrow: direction of the hydrophobic moment). B) Simulations of the Amide I and II bands (p-polarization for angles of incidence of 40° (top) and 60°(bottom) of neuropeptide Y for different tilt angles, φ (defined in the bottom panel), of the α-helix from the surface normal. (reprinted with permission (pending) from  ) (Figure courtesy of Professor Mathias Lösche, Carnegie-Mellon University).
) (Figure courtesy of Professor Mathias Lösche, Carnegie-Mellon University).