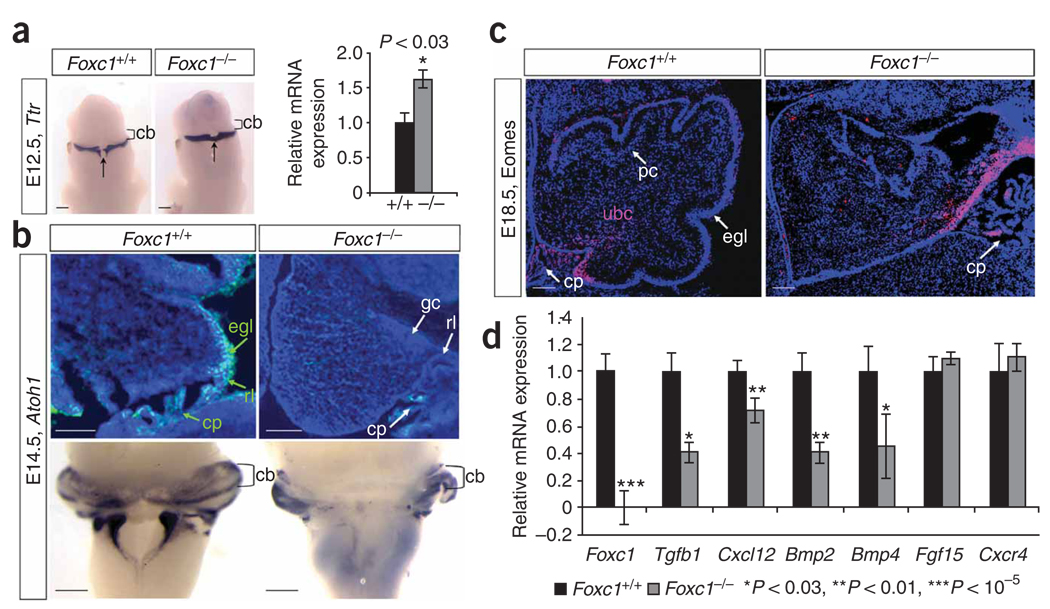
Figure 4.
Loss of Foxc1 from hindbrain mesenchyme disrupts rhombic lip–derived cerebellar cellular populations. (a) Ttr expression in the differentiating choroid plexus epithelium of E12.5 WT and Foxc1 −/− littermate embryos assayed by in situ hybridization (ISH) shows midline expansion of choroid plexus (arrow) adjacent to the cerebellar anlage (cb) in the mutant. Increased Ttr expression confirmed by qRT-PCR (relative to Gapdh expression) is presented as mean ± s.e.m. *P < 0.03 (b) DAPI-counterstained (blue) parasagittal section shows Atoh1 expression (green) in the cerebellar anlage of E14.5 W Tand Foxc1 −/− littermate embryos. No Atoh1+ cells are observed in the mutant egl. Whole-mount ISH of E14.5 brains confirms that loss of Atoh1 in the mutant is restricted to the developing cerebellar vermis. (c) Eomes expression (pink) in the cerebellar anlage of E18.5 WT and Foxc1 −/− littermate embryos. The Eomes+ unipolar brush cells (ubc) remain in the rl of the mutant, rather than migrating through the developing white matter as in WT. DAPI staining (blue) shows egl and pc in WT cerebellum. (d) Expression of six known Foxc1 downstream targets in eye mesenchyme and endothelial development in the mid-hindbrain of E12.5 WT (black) and Foxc1−/− (gray) littermate embryos, assayed by qRT-PCR. Loss of Foxc1 significantly decreases expression of genes secreted from hindbrain mesenchyme (Tgfb1, Cxcl12, Bmp2 and Bmp4) but not genes expressed in the neural tube (Fgf15 and Cxcr4). Data presented as mean ± s.e.m. represent four independent experiments. *P < 0.03, **P < 0.01, ***P< 0.00001. Scale bars, 100 µm.