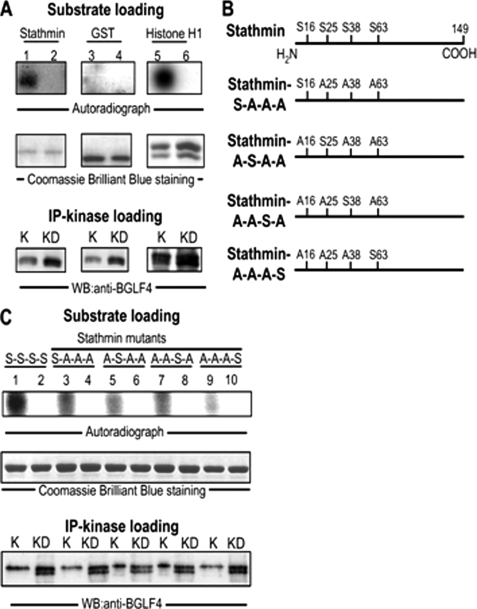
FIGURE 3.
Stathmin is phosphorylated by BGLF4 in vitro. A, recombinant GST-stathmin, GST control protein, and a positive control protein, Histone H1, were used as substrates. HeLa cells were transfected with BGLF4 or BGLF4-kinase dead expression plasmids and BGLF4 (K) and its kinase dead mutant (KD) were obtained by immunoprecipitation with anti-BGLF4 antibody from total cell lysates 24 h post-transfection. IP kinase assays were carried out for 30 min as described under “Experimental Procedures.” The amounts of substrates and kinase loaded are shown. The experiment was performed twice, and a representative example is shown. BGLF4 was observed to phosphorylate GST-stathmin and Histone H1 protein but not GST control protein. B, positions of serine phosphorylation sites in wild-type stathmin and purified stathmin mutants are shown. Phosphorylatable residues of GST-tagged stathmin mutants, which can only be phosphorylated at one of four target residues in the N terminus of stathmin, such as SAAA (only phosphorylatable on residue 16), ASAA (only phosphorylatable on residue 25), AASA (only phosphorylatable on residue 38) and AAAS (only phosphorylatable on residue 63) are indicated. C, substrates of GST-tagged stathmin mutants in B were used in the same reaction as described in A. BGLF4 can be seen to phosphorylate GST-stathmin mutants mainly at residues 16, 25, and 38. The experiment was performed twice, and a representative example is shown.