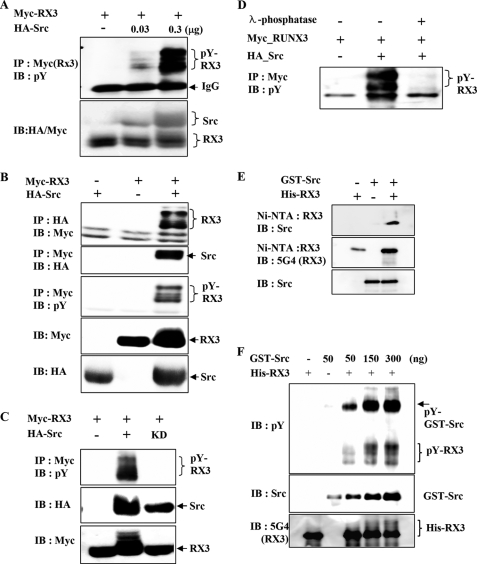
FIGURE 2.
Src kinase phosphorylates RUNX3 on tyrosine residues in vitro and in vivo. A, HEK293 cells were transiently transfected with a fixed amount of Myc-RUNX3 (0.5 μg) and increasing amounts of HA-Src (0, 0.03, and 0.3 μg), and tyrosine phosphorylation of RUNX3 was examined by IP with anti-Myc (RUNX3) antibody and IB with anti-phosphotyrosine antibody (pY; 4G10). B, HEK293 cells were transiently transfected with Myc-RUNX3 and/or HA-Src. Physical interactions between RUNX3 (RX3) and Src were examined by IP and IB using the indicated antibodies (top and 2nd panels). Tyrosine phosphorylation of RUNX3 was analyzed by IP with the anti-Myc antibody and IB with the antibody against phosphotyrosine (4G10; pY) (3rd panel). The levels of Myc-RUNX3 and HA-Src in the transfected cells were measured by IB with the anti-Myc and anti-HA antibodies. C, cells were transfected with Myc-RUNX3 in the presence or absence of HA-Src (wild type) or HA-Src-KD (kinase-dead mutant). Cell lysates were analyzed by IP with the anti-Myc antibody and IB with anti-phosphotyrosine antibody (pY). D, cells were transfected with Myc-RUNX3 in the presence or absence of HA-Src. Cell lysates were treated with λ-phosphatase for 1 h at 30 °C and analyzed by IP and IB with the indicated antibodies. E, GST-Src and His-RUNX3 obtained from E. coli were mixed as indicated, and Ni-NTA pulldown experiments were performed to determine the physical interaction between these two proteins. The interaction was analyzed by IB with the indicated antibodies. F, same proteins were subjected to an in vitro phosphorylation analysis. A fixed amount of His-RUNX3 (500 ng) and increasing amounts of GST-Src (50, 150, and 300 ng) were incubated and analyzed by IB with the anti-RUNX3 (5G4), anti-Src, and anti-phosphotyrosine antibodies. The amount of tyrosine-phosphorylated RUNX3 increased with increasing amounts of Src.