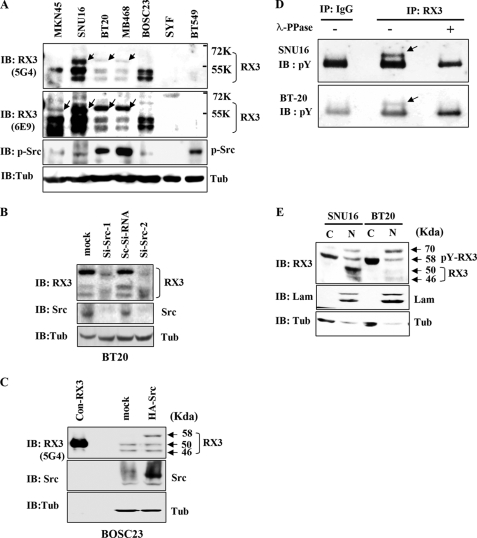
FIGURE 4.
Tyrosine phosphorylation of endogenous RUNX3 in cancer cell lines. A, immunoblotting analysis of whole cell lysates from the indicated cell lines using two different anti-RUNX3 (RX3) antibodies (5G4 and 6E9) and an antibody against phosphorylated-Src (p-Src). An anti-tubulin (Tub) antibody was used as a loading control. Both the anti-RUNX3 antibodies detected a 58-kDa protein (indicated by arrows) in Src-activated cells and in cells with mislocalized RUNX3 (see text for further details). This phosphorylated RUNX3 is larger than the normally detected bands (46 and 50 kDa). Activated Src was detected by the anti-p-Src antibody. B, BT20 cells were grown for 48 h after treatment with scrambled Si-RNA (Sc-Si-RNA) or Src specific siRNA (Si-Src-1, 5′-GCCUCUCAGUGUCUGACUUCGACAA-3′; Si-Src-2, 5′-GCCUCUCAGUGUCUGACUUCGACAA-3′). Cell lysates were immunoblotted with the indicated antibodies. C, BOSC23 cells were transiently transfected with or without HA-Src, and the cell lysates were immunoblotted with the indicated antibodies. Cell lysates were also obtained from BOSC23 cells transfected with RUNX3 (without tag), and small amounts (1:20 compared with other samples) were loaded as molecular weight markers (con-RX3, 50 kDa). D, SNU16 and BT20 cell lysates were treated with or without λ-phosphatase (λ-PPase) for 1 h at 30 °C, then immunoprecipitated with the anti-RUNX3 antibody (6E9; RX3) or IgG as a control, and immunoblotted with the anti-phosphotyrosine antibody (pY). E, nuclear (N) and cytoplasmic (C) fractions were obtained separately from each of the Src-activated cell lines and analyzed by immunoblotting with the anti-RUNX3 antibody (6E9; RX3). Laminin (Lam) and tubulin (Tub) were used as fractionation controls. The 58-kDa protein (tyrosine-phosphorylated RUNX3) was detected mainly in the cytoplasmic fractions.