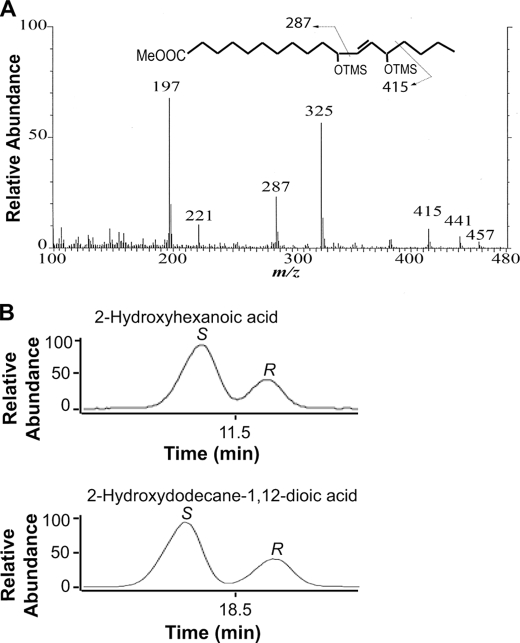
FIGURE 6.
GC-MS analysis of 11,14-DiHOME and steric analysis after ozonolysis. A, electron impact mass spectrum of the trimethylsilyl ether methyl ester derivative of 11,14-DiHOME. The C value was 20.8. B, steric analysis of the hydroxyl groups of 11,14-DiHOME. 11,14-DiHOME was derivatized with (−)-menthoxycarbonyl chloride and subject to ozonolysis, which yielded 2-hydroxyhexanoic and 2-hydroxydodecane-1,12-dioic acid derivatives. Top, the reconstructed ion chromatogram shows that the derivative of 2-hydroxyhexanoic acid had the same retention time as the 2S stereoisomer (co-injection with this derivative of (2R,2S)-hydroxyhexanoic acid). Bottom, the reconstructed ion chromatogram shows that the derivative of 2-hydroxydodecane-1,12-dioc acid had the same retention time as the 2S stereoisomer (co-injection with (2R,2S)-hydroxydodecane-1,12-dioic acid). Analysis of the biological products alone yielded single peaks (data not shown). The results suggest that (11S,14S)-dihydroxy-(12E)-octadecenoic acid was formed. Conditions were as follows: GC oven at 260 °C, isothermal.