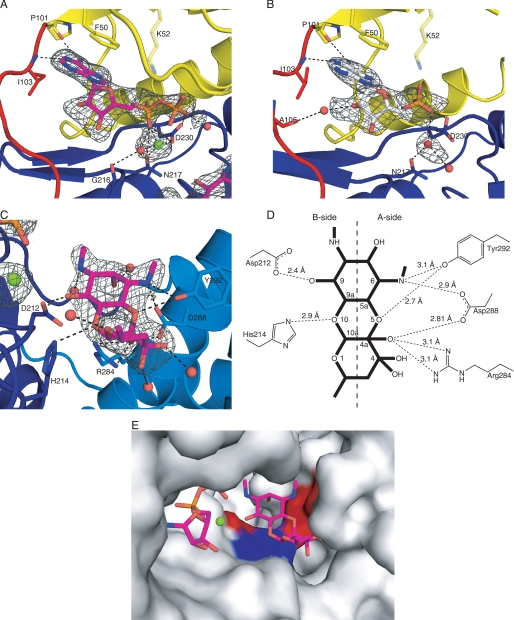
FIGURE 4.
Active site of APH(9)-Ia. A and B, schematic representations of the nucleotide-binding site of the ternary (A) and binary (B) complexes of APH(9)-Ia. ADP is shown as magenta sticks in A and AMP is shown as white sticks in B. The protein is colored according to the assignment in Fig. 2. Interactions between amino acid residues and the nucleotide are shown in dashed lines. Lys-52 is semitransparent because its interaction with the nucleotide cannot be confirmed. Solvent molecules are shown as red spheres and the magnesium ion as a light green sphere. C, schematic representation of the substrate-binding site in the ternary complex of APH(9)-Ia. Spectinomycin is shown in magenta sticks and residues interacting with the substrate and their hydrogen bond interactions with the substrate are indicated as in A and B. D, schematic representation of the hydrogen bonding interactions between spectinomycin and APH(9)-Ia. E, molecular surface of APH(9)-Ia in the vicinity of the active site illustrating the contoured spectinomycin-binding groove. ADP and spectinomycin are shown as magenta sticks. The surface is in light gray and the polar patches surrounding spectinomycin are colored red to represent negatively charged areas and blue to represent positively charged areas. The amino acids that make direct interactions with spectinomycin are mapped to the charged patches.