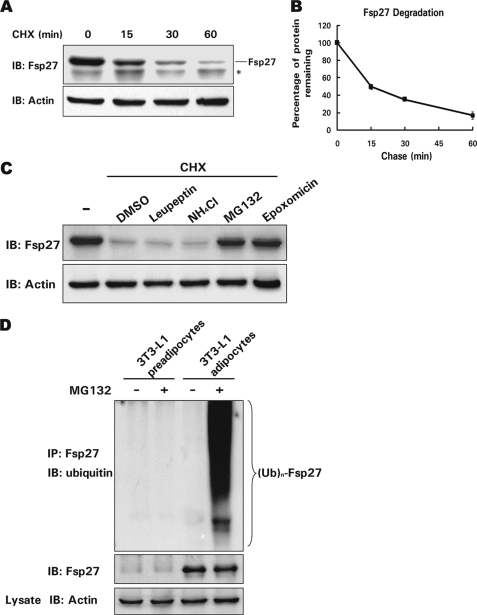
FIGURE 1.
Fsp27 is a short lived protein, and its degradation is dependent on proteasome activity in adipocytes. A, Fsp27 is a short lived protein in adipocytes. Fsp27 stability was evaluated in a cycloheximide (CHX, 100 μg/ml) chasing experiment. CHX was added to the culture medium of 3T3-L1 adipocytes that had been differentiated for 8 days. Cells were harvested 0, 15, 30, or 60 min after the addition of CHX. The Fsp27 protein level was evaluated using Western blot with an antibody against Fsp27. IB, immunoblot. The asterisk designates a nonspecific band recognized by the Fsp27 antibody. β-Actin was used as a loading control. B, quantitative analysis of the Fsp27 level in A using Quantity One software. The Fsp27 level before CHX treatment was designated as 100%. Experiments were repeated three times. C, Fsp27 degradation is dependent on proteasome activity. Differentiated 3T3-L1 adipocytes were pretreated with DMSO, 5 μg/ml leupeptin (an inhibitor of trypsin and cysteine proteases), 10 μm NH4Cl (a general lysosomal protease inhibitor), or 10 μm MG132 or 1 μm epoxomicin (both proteasome-specific inhibitors) for 30 min. CHX was then added to the culture medium for 45 min, and the Fsp27 protein level was evaluated using Western blotting. D, Fsp27 is polyubiquitinated. Fsp27 from differentiated 3T3-L1 adipocytes treated with or without MG132 (10 μm) for 2 h was immunoprecipitated with an antibody against Fsp27. The level of Fsp27 ubiquitination was detected using an antibody against ubiquitin. Untreated 3T3-L1 preadipocytes or preadipocytes treated with MG132 were used as negative controls for Fsp27 expression and ubiquitination. IP, immunoprecipitation. (Ub)n-Fsp27 denotes polyubiquitinated Fsp27.